
Prolonged albumin administration in patients with decompensated cirrhosis: the amount makes the difference
Systemic inflammation and cardiovascular dysfunction represent the main pathophysiological features of decompensated cirrhosis. The “peripheral vasodilation hypothesis” published in 1988 (1) has identified in the arterial vasodilation, mainly occurring in the splanchnic area, the primary cause of the effective hypovolemia, which characterizes the cardiovascular status of patients with decompensated cirrhosis. Few years later, a relative decline of cardiac output favored by cirrhotic cardiomyopathy has been also advocated as an additional event aggravating effective hypovolemia in the more advanced stages of the disease (2). Besides cardiovascular dysfunction, patients with decompensated cirrhosis present a sustained pro-inflammatory and pro-oxidant state generated by a complex network of closely inter-related pathways. The “systemic inflammation hypothesis” published in 2014 postulates that this condition is caused by the continuous stimulation of the immune system cells by the pathogen associated-associated molecular patterns (PAMPs) activated by the translocation of bacterial products from the intestinal lumen to the circulation as a result of several alterations initiated and favored by portal hypertension (i.e., quantitative and qualitative changes in gut microbiota, impairment in intestinal mucosal barrier, increased epithelial permeability, and impaired intestinal immunity) (3). Similar mechanisms are also triggered by the systemic spread of danger-associated molecular patterns (DAMPs) from the diseased liver where inflammation and cell death take place (3). Systemic inflammation can promote arterial vasodilation by inducing local release of nitric oxide and other vasoactive molecules or favor cardiac dysfunction through the depression of heart muscle contractility (4). However, several evidences indicate that the direct consequences of systemic inflammation on immune function and cellular metabolism represent the predominant pathogenetic mechanism of organ dysfunction in patients with decompensated cirrhosis, particularly in those who develop acute-on-chronic liver failure (ACLF) (5).
As a result of this improved knowledge, the identification of treatments able to interrupt the pathophysiological cascade leading to organ failure has become a major goal of research in decompensated cirrhosis.
Among all the potential treatments, human albumin (HA) is the agent with more translational and clinical supporting evidences. Besides being the main modulator of fluid distribution in the various body compartments since it accounts for about 70–80% of the plasma oncotic pressure, HA is provided of many other biological properties, which rely on the peculiar structure and conformation of the molecule (6). HA binds and carries a long variety of endogenous and exogenous substances, thus exerting an essential role of transport and detoxification. It is the major circulating antioxidant in the body able to scavenge large amounts of reactive oxygen and nitrogen species. HA also contributes to the integrity of the vessels by stabilizing the endothelia. Finally, HA modulates the inflammatory and immunological responses through different mechanisms (7,8).
HA has been traditionally given to patients with decompensated cirrhosis with the aim to improve effective volemia due to its capacity of expanding plasma volume (9,10). However, based on its non-oncotic properties, HA can target several different steps of the pathophysiological network linking systemic inflammation, cardiovascular dysfunction and organ failure in decompensated cirrhosis. Accumulating experimental and clinical evidences support this hypothesis. In rats with carbon tetrachloride-induced cirrhosis and ascites, the administration of HA is able to restore cardiac contractility by antagonizing the effects of pro-inflammatory cytokines and oxidative stress in the heart tissue (7). Furthermore, the improvement of systemic hemodynamics observed in patients with spontaneous bacterial peritonitis receiving HA is associated to a striking rise in peripheral vascular resistances rather than to an increase in cardiac index, suggesting an effect of HA on endothelial function rather than as plasma-expander (11). Finally, HA supplementation binds circulating prostaglandin E2 (PGE2), which has an immunosuppressive activity and is several folds elevated in patients with decompensated cirrhosis, thus contributing to immune competence (8). Therefore, in a pathophysiological perspective, prolonged HA administration has been proposed as a multi-target therapy for the management of patients with decompensated cirrhosis (12).
Following two pivotal randomized trials showing a better control of ascites with HA (13,14), three clinical trials evaluating the effects of long-term HA administration to patients with decompensated cirrhosis have been published in 2018 (15-17). In the ANSWER study (15), a non-profit, multicenter, randomized, open-label, pragmatic trial, 431 patients with persisting non complicated ascites requiring the administration of at least 200 mg of anti-mineralocorticoids and 25 mg of furosemide per day were randomized to either standard medical treatment (SMT) or SMT plus 40 g of albumin (HA) twice a week for the initial two weeks and then 40 g once a week. A significantly better 18-month overall survival was seen in patients receiving HA, with a 38% reduction of the hazard ratio for mortality. Furthermore, the cumulative incidence of paracentesis, refractory ascites and other severe complications of cirrhosis was significantly reduced.
The core results of the ANSWER trial have been very recently confirmed by a prospective, non-randomized clinical trial, which enrolled 70 patients with cirrhosis and refractory ascites (16). Patients who received SMT + HA (20 g twice a week) had a significantly lower 24-month mortality than the patients receiving the SMT.
A contemporary placebo controlled clinical trial “midodrine-albumin in cirrhotic patients awaiting liver transplantation” (MACHT trial) obtained different results (17). In this trial, 173 patients with ascites listed for liver transplantation were randomized to receive SMT plus 40 g of HA every 15 days and the α1-receptor agonist midodrine or SMT plus placebos. No differences were seen in the probability of developing complications or death during the 12 months of follow-up.
Although the differences in the design of the studies, types of patients enrolled and amount and schedule of HA infused likely explain the divergent results, additional information are eagerly awaited if we consider the impact that this new strategy would have in the management of patients with decompensated cirrhosis.
The study published in Gastroenterology by Fernández and co-authors (18), based on the results of two trials (the Pilot-PRECIOSA and the INFECIR-2), provides novel important information related to the effects of prolonged HA administration in patients with decompensated cirrhosis, which are highly relevant for both the implementation of this new approach in the daily clinical practice and the identification of the mechanisms through which HA exerts its beneficial effects (18).
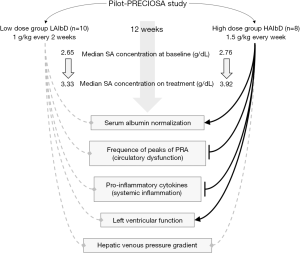
The Pilot-PRECIOSA (18) was an open-label, non-randomized, prospective study aimed to get preliminary data for the design of a currently ongoing multicenter randomized therapeutic trial, which evaluate the efficacy of long-term (1 year) HA treatment in the prevention of ACLF and mortality (PRECIOSA study, ClinicalTrials.gov: NCT03451292). Specifically, the Pilot-PRECIOSA study was aimed to identify the HA dosage able to normalize serum albumin (SA) concentration (3.4–4.7 g/L) during 12 weeks of treatment and improve cardiocirculatory dysfunction and systemic inflammation.
The study enrolled non-infected patients with decompensated cirrhosis and severe circulatory dysfunction, as defined by the presence of ascites, renal dysfunction [serum creatinine ≥1.2 mg/dL or blood urea nitrogen ≥25 mg/dL or dilutional hyponatremia (serum sodium ≤130 mEq/L)], high levels of plasma renin activity (PRA) (≥2 ng/mL.h), and need for diuretic treatment to prevent ascites recurrence (at least 200 mg of spironolactone or 100 mg of spironolactone plus 40 mg of furosemide).
The first 10 patients received 1 g/kg body weight of albumin every 2 weeks [low-albumin dose (LAlbD) group]. An interim analysis showed that this dose of HA was insufficient to normalize SA concentration in all patients except one, with the SA concentration moving from a pre-treatment median level of 2.65 g/dL to a median value on treatment of 3.33 g/dL. As a result of this finding, the HA dosage and the frequency of its administration were increased to 1.5 g/kg body weight every week in the following 8 patients [high-albumin dose (HAIbD) group]. All the 6 patients of the HAlbD group presenting baseline hypoalbuminemia normalized SA concentration “on treatment” (P<0.001). Overall, SA concentration rose from a pre-treatment median level of 2.76 g/dL to a median value during treatment of 3.92 g/dL.
An interesting finding of the pilot-PRECIOSA study was that the degree of circulatory dysfunction and systemic inflammation is extremely unstable in patients with advanced cirrhosis, as they presented intense, acute, high and reversible peaks in circulating PRA and interleukin-6 (IL-6) levels even in absence of any clearly evident precipitating event. Both LAlbD and HAlbD did not produce significant suppression in PRA levels, although peaks were observed more frequently in the LAlbD group than in the HAlbD one. Interestingly, prolonged treatment with HAlbD, but not with LAlbD, was associated to a significant increase in cardiac index, systolic volume and left ventricular stroke work index, thus indicating an improvement of left ventricular function.
Furthermore, the HAlbD, but not LAlbD, induced a significant reduction (>20%) of IL-6 concentration. This latter finding prompted to extend the investigation to additional 13 pro-inflammatory cytokines chosen among an initial panel of 24 because found significantly higher in patients with decompensated cirrhosis than healthy subjects. The results clearly indicated that only treatment with HAlbD was associated with a marked suppression of most of pro-inflammatory cytokines.
To confirm these results the investigators also analyzed the blood samples collected during the INFECIR-2 study, which was an investigator-driven, multicenter randomized clinical trial assessing, in patients with cirrhosis and acute bacterial infections unrelated to spontaneous bacterial peritonitis, the effect of short-term albumin (1.5 g/kg at diagnosis and 1 g/kg on the third day) on top of antibiotic therapy. Again, a significant reduction in plasma renin concentration and circulating plasma cytokines was observed only in patients receiving HA plus antibiotics as compared to those treated with antibiotics alone.
Three major results emerge from this study by Fernández and coauthors: (I) the beneficial effects of prolonged HA administration are not mediated only by the improvement of cardiocirculatory dysfunction, but also by the attenuation of systemic inflammation; (II) these effects are dose-dependent, as they were obtained only by using the HAlbD; and (III) the beneficial effects of HA occurs only when the median on-treatment SA concentration reaches the normal range.
The first consequence of these results is that HA should be considered in patients with decompensated a drug rather than only a plasma expander. Indeed, it appears logical to assume that its anti-inflammatory activity is mediated by the biological properties of the molecule unrelated to the oncotic power. Although the cellular mechanisms are still undetermined, preliminary results have recently unveiled that HA, after being internalized by immune cells, is able to block the activation of pro-inflammatory receptors (19).
A second important consequence is that the dose of HA infused is important to achieve the beneficial effects of treatment, indicating that the use of low doses may result in a futile treatment. And indeed, the HA administered in the MACHT trial (17) was half with respect to the amount given to the patients enrolled in the trial on refractory ascites (16) and even less in those enrolled in the ANSWER trial (15), who also received a loading dose of 80 grams per week in the initial 2 weeks (Table 1).

Full table
If this is the case, the question is how can we judge in the daily clinical practice whether the dose of HA is adequate? Again, the results of the study from Fernández and co-authors provide useful information to answer this question. An essential observation is that only the HAlbD was able to normalize the SA concentration, to a median level during treatment close to 4 g/dL. Interestingly, a post-hoc analysis of the ANSWER trial recently presented at the 2019 ILC congress showed that SA levels reached after 1 month of treatment were strongly associated with the probability of 18-month survival, which was greater than 90% in those patients reaching a level of 4 g/dL (20). Thus, it could be speculated that treatment with HA is able to produce relevant effects only if administered at doses able to significantly increase SA concentration to reach normal levels.
Finally, the present study indicates that the administration of HAlbD is safe even in patients with advanced decompensated cirrhosis, as no cases of volume overload or complications related to portal hypertension were reported (18). At this regard, treatment was not associated with significant changes in hepatic venous pressure gradient. However, it should be kept in mind that a word of caution should be still given when high dose of HA are administered in patients at risk of volume overload (heart failure, pulmonary hypertension, severe pneumonia) (21) and in those with gastro-esophageal varices at high risk of bleeding.
In conclusion, the study by Fernández and colleagues (18) shows that prolonged HA treatment attenuates systemic inflammation and improves cardiocirculatory dysfunction and provides useful hints on how to administer HA in terms of schedule (dose and frequency) of administration with the aim to reach an on-treatment SA concentration within the normal range (Figure 1). Although the regular intravenous HA infusions require frequent utilization of healthcare services and careful patient compliance, these results further support the clinical advantages of this novel approach for patients with decompensated cirrhosis and justify the care management needed for long-term HA administration, thus encouraging a change of the current indications for HA treatment from only targeting specific complications to a more comprehensive approach able to globally reduce the incidence of life-threatening complications and improve patient survival, provided that HA is given in a sufficient amount and for a sufficient length of time.
Acknowledgments
None.
Footnote
Conflicts of Interest: G Zaccherini is part of the speakers’ bureau for Octapharma. P Caraceni is part of the speakers’ bureau for Grifols SA, Octapharma AG, Baxalta, Alfa Sigma, Takeda and Kedrion Biopharma, he is consultant for Kedrion Biopharma, he is on the advisory board for Grifols SA and received a research grant from Octapharma AG. M Tufoni has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151-7. [Crossref] [PubMed]
- Møller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J 2013;34:2804-11. [Crossref] [PubMed]
- Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272-84. [Crossref] [PubMed]
- Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 2006;43:S121-31. [Crossref] [PubMed]
- Clària J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249-64. [Crossref] [PubMed]
- Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836-46. [Crossref] [PubMed]
- Bortoluzzi A, Ceolotto G, Gola E, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology 2013;57:266-76. [Crossref] [PubMed]
- O’Brien AJ, Fullerton JN, Massey KA, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med 2014;20:518-23. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651-3. [Crossref] [PubMed]
- Fernández J, Monteagudo J, Bargallo X, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology 2005;42:627-34. [Crossref] [PubMed]
- Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol 2018;15:753-64. [Crossref] [PubMed]
- Gentilini P, Casini-Raggi V, Di Fiore G, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol 1999;30:639-45. [Crossref] [PubMed]
- Romanelli RG, La Villa G, Barletta G, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol 2006;12:1403-7. [Crossref] [PubMed]
- Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417-29. [Crossref] [PubMed]
- Di Pascoli M, Fasolato S, Piano S, et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int 2019;39:98-105. [Crossref] [PubMed]
- Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol 2018;69:1250-9. [Crossref] [PubMed]
- Fernández J, Clària J, Amorós A, et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology 2019;157:149-62. [Crossref] [PubMed]
- Duran-Güell M, Casulleras M, Flores-Costa R, et al. Albumin protects the liver from tumour necrosis factor alpha- induced cell death. J Hepatol 2019;70:e92. [Crossref]
- Caraceni P, Riggio O, Angeli P, et al. Serum albumin concentration as guide for long-term albumin treatment in patients with cirrhosis and uncomplicated ascites: Lessons from the ANSWER study. J Hepatol 2019;70:e53. [Crossref]
- Thévenot T, Bureau C, Oberti F, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol 2015;62:822-30. [Crossref] [PubMed]


