
Novel therapeutic approaches for pancreatic cancer by combined targeting of RAF→MEK→ERK signaling and autophagy survival response
KRAS (Kirsten rat sarcoma viral oncogene homolog) is a major target in pancreatic ductal adenocarcinoma (PDAC), but so far it has been perceived as “undruggable”, due to a lack of effective inhibitors. Kinsey et al. (1) have recently reported that inhibition of the RAF (rapidly accelerated fibrosarcoma)→MEK (MAPK/ERK kinase)→MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) signaling pathway, downstream of KRAS, elicits autophagy as a survival response, thus protecting PDAC cells from the cytotoxic effects of RAF→MEK→ERK inhibition. MEK1/2 inhibition promotes activation of the LKB1→AMPK→ULK1 signaling axis, thus leading to pancreatic cancer cells to mount a protective survival autophagy response. This might explain, at least in part, the lack of clinical benefit of MEK1/2 inhibitors (e.g., trametinib, pimasertib) in PDAC patients, as assessed by the absence of a statistically significant improvement in overall survival (2,3), and might suggest a treatment strategy, involving inhibition of both RAF→MEK→ERK signaling and autophagy, for PDAC and other RAS-driven cancers (1). This is of importance because there is an urgent need to establish new frameworks to improve future treatments as, despite intensive research over the past several years, prognosis of PDAC remains gloomy, with no effective therapeutic treatment and with median survivals of less than a year.
Pancreatic cancer
Pancreas is made of two major functional compartments, exocrine and endocrine, and it is composed of three critical cell lineages: islet (endocrine), acinar, and ductal (4). Most of the pancreas is made up of exocrine cells, which form the exocrine glands and ducts. The exocrine pancreas comprises acinar, ductal and centroacinar cells, producing and secreting enzymes that aid to digest food. Ductal cells form an intricate network of small tubes called ducts through which the digestive enzymes (such as lipases, proteases, amylases) secreted by acinar cells flow. These ducts carry the digestive juices into the main pancreatic duct, which merges with the common bile duct (carrying bile from the liver) and drains its fluid into the duodenum at the ampulla of Vater to break down fats, proteins and carbohydrates, thus helping food digestion. Pancreatic acinar cells have the intrinsic ability and plasticity to undergo transdifferentiation to a progenitor-like cell type with ductal characteristics, a process termed as acinar-to-ductal metaplasia, occurring during pancreatitis and may represent an initial step towards pancreatic ductal adenocarcinoma (5,6). The endocrine pancreas is composed of small islands of specialized cells called the islets of Langerhans that make and secrete hormones. The endocrine cells produce and release hormones (such as insulin and glucagon) into the bloodstream, thus controlling blood sugar (glucose) levels. Most tumors affecting the exocrine gland are called adenocarcinomas. The vast majority of pancreatic cancer (about 95% of pancreatic cancers) involves the exocrine pancreas and initiates in the ducts of the pancreas when the exocrine cells start to grow out of control, thus leading to the name of pancreatic ductal adenocarcinoma (PDAC) for the most common malignancy of the pancreas.
Only a small percentage (1–2%) of all pancreatic cancers correspond to slower-growing pancreatic neuroendocrine tumors (PanNETs), previously known as islet cell tumors, which have a slow, indolent growth and are asymptomatic (7). Because PanNETs affect the secretion of hormones, they are named after the hormone they secrete (gastrinoma, insulinoma, somatostatinoma, VIPoma, and glucagonoma, affecting cells making gastrin, insulin, somatostain, VIP and glucagon, respectively). PanNETs, which are much less common than pancreatic exocrine tumors, have a better prognosis than PDAC, with an overall median survival from diagnosis of 4.1 years, which is considerably longer than the 6-month median for PDAC (8).
PDAC is the most lethal of all common cancers, with the highest mortality-to-incidence ratio (Figure 1), being an indolent tumor difficult to treat that shows a rapid progress from diagnosis to death. This is in part due to the fact that PDAC goes undetected until it becomes symptomatic, and for this reason the tumor is usually locally advanced or metastatic at the time of diagnosis. Complete surgical resection remains the only potential curative treatment, but only 10–20% of pancreatic cancers are resectable at the time of diagnosis, and even the 5-year survival rate for PDAC after surgery remains rather low (15–20%), mainly due to metastatic disease or local recurrence (10).

PDAC shows an overall 5-year survival rate of less than 5–8%, depending on the specific stage of disease when it is diagnosed (11-14). Because of difficulties in early diagnosis, the occurrence of metastases before clinical detection, the aggressiveness of the tumor, and the lack of effective therapies, PDAC shows a poor prognosis. The incidence and mortality rates for PDAC are nearly equivalent (Figure 1), and the median survival in metastatic pancreatic cancer is nearly 6 months. Globally pancreatic cancer shows the highest mortality-to-incidence ratio of all cancers (Figure 1), with incidence rates highest in North America, Europe, and Australia/New Zealand. Currently, pancreatic cancer is the seventh highest cause of death from cancer worldwide, if individual cancer sites are considered (9), or the ninth cause of cancer death if we include colorectal, hematological and head and neck cancers as additional cancer types that encompass a variety of tumors in similar or nearby locations (Figure 1). The incidence rate of pancreatic cancer is rising, but prognosis remains extremely poor. As a result, pancreatic cancer is estimated to become the third leading cause of death from cancer in the European Union after lung and colorectal cancers in the coming future (15), and the second leading cause of cancer-related death in the United States by 2030 (16).
There are clear differences between the genetic landscapes of PADC and PanNETs. More than 90% of PDAC cases at all grades carry a faulty KRAS gene, and none of the most commonly mutated genes in PDAC [KRAS, CDKN2A (encoding p16), TP53 and SMAD4] are currently druggable (17). In contrast, KRAS mutation is normally absent in PanNETs, which show 60% fewer genes mutated per tumor than in PADCs. The above genes most commonly affected by mutation in PDACs are rarely altered in PanNETs and viceversa (18). Genes that are frequently mutated in PanNET include MEN1, DAXX, ATRX and mTOR (18,19).
Lack of efficiency of current therapy in the treatment of pancreatic cancer
PDAC is the epitome of a treatment-resistant malignancy, driven by a so far “undruggable” oncoprotein, KRAS (20,21). Pancreatic cancer is a major cause of cancer-associated mortality, with a dismal overall prognosis that has remained virtually unchanged for many decades. At the time of diagnosis for pancreatic cancer, about 15% of patients have resectable disease (stage I or II), 35% locally advanced pancreatic cancer (stage III), and 50% metastatic disease (stage IV) (22). Palliative gemcitabine has been the standard treatment for pancreatic cancer for many years with a modest survival benefit of about 3 months. At present the first-line therapy in pancreatic cancer includes FOLFIRINOX (made up of: folinic acid, 5-fluorouracil, irinotecan and oxaliplatin) and nab-paclitaxel plus gemcitabine, whereas combinations of gemcitabine plus cisplatin and temsirolimus plus bevacizumab are used for second-line treatment, but in all cases the survival outcomes of pancreatic cancer remain poor (21,23). Thus, PDAC remains one of the most lethal malignancies with a gloomy prognosis, and therefore new therapeutic drugs and approaches are urgently needed. Unfortunately, the failure rate of phase III clinical trials in PDAC is very high (87%) (24), likely due to the lack of robustness of the preclinical studies underpinning clinical trials, which overlook major variables and players and use rather simple and/or inadequate models.
KRAS and MEK→ERK signaling in pancreatic cancer
Activating mutations in KRAS are a hallmark in PDAC, occurring in 90–95% cases of the deadly and highly metastatic PDAC (25-27). Additional frequently mutated genes also include TP53, SMAD4, CDKN2A, ARID1A, ROBO2, PREX2, BRCA2 and MLL3 (25,28). KRAS encodes a small GTPase that is activated through binding of GTP and translocation to the plasma membrane, cycling between an active GTP-bound form and an inactive GDP-bound form. The majority of KRAS mutations occur at codons 12, 13 and 61, leading to constitutive activation, as the protein becomes insensitive to GTPase-activating proteins (GAPs), which induce GTP hydrolysis to GDP and turn RAS into its inactive form. The involvement and driver role of KRAS oncogenic activation in PDAC has been firmly established by using genetically engineered mouse models (28). This makes KRAS an attractive therapeutic target. However, despite more than three decades of research effort, no effective pharmacological inhibitors of KRAS have reached the clinic, leading to the widely held perception that KRAS protein may be “undruggable” (20).
KRAS signals through a series of downstream pathways, with the so called RAF→MEK→ERK and phosphoinositide-3-kinase (PI3K)→AKT→mTOR signaling routes, which show extensive cross-talk, as the major RAS downstream signaling pathways. Figure 2 shows a schematic view of these signaling pathways and their interactions via cross-inhibition and cross-activation.
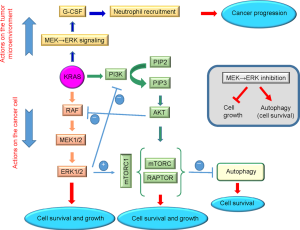
Because oncogenic KRAS remains “undruggable” and engages the downstream RAF→MEK→ERK and PI3K pathways, promoting enhanced cellular proliferation, survival and motility, a putative way to treat these KRAS-driven cancers could involve the inhibition of KRAS downstream signals, such as the MEK→ERK and/or PI3K pathways, as single or combinatorial therapeutic strategies. This approach acquires a high relevance when KRAS, despite being a hallmark in PDAC, can also be dispensable in a subset of PDAC cells, where PI3K pathway activation may bypass the requirement for KRAS (29). Although PI3K has typically been considered a RAS effector, a growing body of evidence suggests that PI3K can act upstream to stimulate RAS→ERK signaling in various contexts (30). Loss of oncogenic KRAS expression led to PI3K-dependent ERK signaling, and sensitivity to PI3K inhibitors, showing an alternative bypass mechanism through canonical (i.e., AKT signaling) and non-canonical (i.e., ERK signaling) PI3K signaling (29). The mechanism of how PI3K stimulates wild-type RAS→ERK activation in oncogenic KRAS deficient PDAC cells remains unclear (29), but it could involve phospholipid second messengers (31).
On the other hand, KRAS activation on cancer cells extends to the surrounding microenvironment, and also leads to recruitment of neutrophils (32,33) (Figure 2), which seem to play a major role in cancer development (34). A RAS→RAF→MEK→ERK signaling pathway, leading to G-CSF (granulocyte colony-stimulating factor) upregulation, might be involved in this neutrophil recruitment (35).
Autophagy, survival and cancer
Cancer cells often outstrip their local nutrient supply and they usually face periods of nutrient deprivation during metastasis. Under these conditions, tumor cells should adapt to these new challenging conditions of nutrient stress, involving the multifunctional roles of kinases and phosphatases that modulate downstream signaling pathways to reprogram cellular functions and promote survival (36). A prominent strategy for cells to scavenge energy is through the initiation of autophagy by phosphorylation of ULK1 (Unc-51-like kinase-1) (37). The diverse metabolic fuel sources generated by autophagy provide the metabolic plasticity required for tumor cells to survive and thrive in stringent microenvironments and under starvation conditions (38).
Though autophagy has long been linked, in certain settings, to a form of cell death, initially named type II cell death to be distinguished from type I (apoptotic) cell death and later called autophagic cell death (39), its major role is associated with a pro-survival stress response (40). In this regard, there is increasing evidence of a potential role for autophagy in tumor growth and resistance to chemotherapy (41). A subpopulation of dormant tumor cells surviving KRAS ablation and responsible for tumor relapse has been reported to rely on autophagy and mitochondrial function for survival (42).
Autophagy seems to act as a safe haven for cancer cell survival against nutrient starvation, metabolic stress, hypoxia and chemotherapy-induced cell death. This reminds other situations occurring in normal cells, including the immune system, in which autophagy is critical to keep cells alive under stressful conditions. Depletion of the amino acid L-Arg leads to a reversible response that preserves T lymphocytes through endoplasmic reticulum stress and autophagy, while remaining arrested at G0/G1 cell cycle phase, but the endoplasmic reticulum stress response leads to apoptosis when autophagy was inhibited (43). This highlights the essential role of autophagy as a cytoprotective response to endoplasmic reticulum stress (43). Increasing evidence supports endoplasmic reticulum stress as a potent trigger for autophagy, this latter acting as an adaptive response (44). Interestingly, the endoplasmic reticulum has also been shown to be a promising target in pancreatic cancer, and an endoplasmic stress response might lead to the triggering of apoptosis (45). Thus, it is tempting to envisage that an autophagy response could also be triggered following endoplasmic reticulum stress, and thereby a putative combination therapy, including induction of endoplasmic reticulum stress and autophagy inhibition, could be applied for new treatment approaches.
Taken together, accumulating evidence highlights the crucial role of autophagy as a survival signal, this being especially relevant in cancer cells, and thereby autophagy has become a promising therapeutic target for cancer treatment. In this regard, there is mounting preclinical evidence showing that autophagy targeting can potentiate the efficacy of several anticancer therapies (41,46). The 4-aminoquinoline agents chloroquine and hydroxychloroquine, used for decades against malarial infections, and later also to treat systemic lupus erythematosus and rheumatoid arthritis, are classical autophagy inhibitors, and several encouraging preclinical and clinical data support and warrant further studies on their potential as anti-cancer agents (47).
Combined treatment of MEK→ERK and autophagy inhibitors to kill PDAC cells
As stated above, KRAS mutations are known to be a driver event of PDAC, but targeting mutant KRAS has proved challenging. Because targeting oncogene-driven signaling pathways is a clinically validated approach for several devastating diseases, an appealing therapeutic approach is targeting the KRAS downstream signaling pathways, such as the RAF→MEK→ERK signaling route. In this context, Kinsey et al. found that xenografts in NOD/SCID mice of human pancreatic cancer cell lines (Mia-PaCa2 and BxPC3) or tumor tissue obtained from PDAC patients were rather resistant to single agent trametinib (MEK inhibitor) or chloroquine/hydroxychloroquine (autophagy inhibitor), but were highly sensitive to the combination of both inhibitors (1). In addition, a partial disease response was achieved following the combination treatment of trametinib plus hydroxychloroquine in a patient with metastatic PDAC refractory to standard-of-care therapies, including neo-adjuvant mFOLFIRINOX, adjuvant gemcitabine/capecitabine and palliative gemcitabine/abraxane/cisplatin (1). These results are totally consistent with those reported by Bryant et al. (48), published as a companion manuscript in the same issue of Nature Medicine. These authors found that autophagy inhibitor chloroquine and genetic or pharmacologic inhibition of autophagy regulators enhanced the ability of ERK inhibitors to mediate antitumor activity in KRAS-driven PDAC (48). Taking together, these data show compelling evidence that inhibition of ERK signaling pathway drives PDAC cells to become acutely dependent on autophagy, thus becoming highly sensitive to autophagy inhibitors.
The results reported by Kinsey et al. (1) are also consistent with previous observations that autophagy serves as an adaptive and protective response to inhibition of RAS→RAF→MEK→ERK signaling in cancer (41). Autophagy is particularly active during metabolic stress, a process that often occurs in solid tumors and tumor microenvironment (38). ERK inhibition leads to a limited degree of apoptosis in KRAS-mutant pancreatic cancer cells (49), but cell death is significantly increased by the combined inhibition of ERK and autophagy (48). These data are further supported by additional evidences where the cell death response in PDAC cells induced by inhibition of survival ERK and NF-κB signaling routes was promoted by the concomitant inhibition of autophagy (50). Using a combinatorial siRNA platform, the oncogenic KRAS signaling has been shown to be mediated by multiple pathways, highlighting RAF→MEK→ERK and autophagy pathways as main routes to keep cancer cell survival (51). The identification of autophagy as a major target for cancer treatment is a valuable insight, as autophagy plays a major role in tumor development, and promotes tumor growth and survival by supporting tumor metabolism (38).
MEK→ERK signaling as a switch for different cell death/survival processes
Induction of cell death following chemotherapeutic agents seems to involve the participation of triggers, initiators, mediators and executioners (52), with a complex network of interactions and intersections like a railway junction where different rails or signaling routes converge and diverge. In this context, MEK→ERK signaling pathway seems to be a major player in this signaling junction.
ERK1/2 has been found to act as a switch between necroptotic and apoptotic cell death (53). ERK1/2 behaves as a regulator of the type of cell death occurring as a result of the action of the proapoptotic ether lipid edelfosine in glioblastoma cells (53). ERK1/2 activation diverts the cytotoxic action of the ether lipid to necroptotic or survival responses; however inhibition of MEK→ERK signaling pathway potentiates edelfosine-induced apoptosis in glioblastoma cells, thus switching the type of edelfosine-induced cell death from necrosis to apoptosis (53). Inhibition of MEK→ERK signaling also highly potentiates the apoptotic action of additional antitumor agents, including the alkaloid berberine in melanoma cells (54). Thus, MEK→ERK signaling appears to constitute part of a major signaling junction in which different routes leading to apoptosis, necroptosis or autophagy responses converge or diverge, seemingly dependent on the cell type.
Concluding remarks
The recent results reported by Kinsey et al. (1), together with those reported by Bryant et al. (48) in the same April issue of Nature Medicine, support an appealing framework for a treatment approach of the so far intractable PDAC, which could be extrapolated to additional RAS-driven cancers. Inhibition of MEK→ERK signaling leads to a high dependence of PDAC cells on autophagy for survival. This process, by which cancer cells are forced and driven to highly depend on autophagy for survival, is of major importance as autophagy, in this way, becomes a major target for cancer therapy. The combination therapy of MEK→ERK signaling inhibitors and autophagy blockers leads to the killing of PDAC cells, thus rekindling the potential use of autophagy inhibitors, a once written-off strategy, against tumors that are highly dependent on autophagy. Because the two processes affected in this approach, MEK→ERK signaling and autophagy, play critical roles in most cell types, either normal or malignant cells, caution should be taken when extrapolating bench data to the clinical setting. The results of the study conducted by Kinsey et al. (1) constitute a proof-of-concept for the putative therapeutic potential of drugs targeting MEK→ERK signaling and autophagy in PDAC and RAS-driven tumors, and warrant further investigation as an attractive combination therapy. Furthermore, as stated above, additional processes can also promote a high dependency of tumor cells on autophagy, thus offering further new targets to be combined with autophagy inhibitors in cancer treatment.
Acknowledgments
Funding: F Mollinedo and C Gajate are supported by the Spanish Ministry of Science, Innovation and Universities (SAF2017-89672-R).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Kinsey CG, Camolotto SA, Boespflug AM, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med 2019;25:620-7. [Crossref] [PubMed]
- Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 2014;50:2072-81. [Crossref] [PubMed]
- Van Cutsem E, Hidalgo M, Canon JL, et al. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int J Cancer 2018;143:2053-64. [Crossref] [PubMed]
- Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest 2011;121:4572-8. [Crossref] [PubMed]
- Pelosi E, Castelli G, Testa U. Pancreatic Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Biomedicines 2017;5:65. [Crossref] [PubMed]
- Storz P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2017;14:296-304. [Crossref] [PubMed]
- Kelgiorgi D, Dervenis C. Pancreatic neuroendocrine tumors: the basics, the gray zone, and the target. F1000Res 2017;6:663. [Crossref] [PubMed]
- Brooks JC, Shavelle RM, Vavra-Musser KN. Life expectancy in pancreatic neuroendocrine cancer. Clin Res Hepatol Gastroenterol 2019;43:88-97. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ahrendt SA, Pitt HA. Surgical management of pancreatic cancer. Oncology (Williston Park) 2002;16:725-34; discussion 734, 736-8, 740, 743.
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ 2012;344:e2476. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol 2016;55:1158-60. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [Crossref] [PubMed]
- Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. [Crossref] [PubMed]
- Chan CS, Laddha SV, Lewis PW, et al. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 2018;9:4158. [Crossref] [PubMed]
- Papke B, Der CJ. Drugging RAS: Know the enemy. Science 2017;355:1158-63. [Crossref] [PubMed]
- Hajatdoost L, Sedaghat K, Walker EJ, et al. Chemotherapy in Pancreatic Cancer: A Systematic Review. Medicina (Kaunas) 2018;54:48. [Crossref] [PubMed]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163-72. [Crossref] [PubMed]
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-10. [Crossref] [PubMed]
- Thota R, Maitra A, Berlin JD. Preclinical Rationale for the Phase III Trials in Metastatic Pancreatic Cancer: Is Wishful Thinking Clouding Successful Drug Development for Pancreatic Cancer? Pancreas 2017;46:143-50. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015;6:6744. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Mann KM, Ying H, Juan J, et al. KRAS-related proteins in pancreatic cancer. Pharmacol Ther 2016;168:29-42. [Crossref] [PubMed]
- Muzumdar MD, Chen PY, Dorans KJ, et al. Survival of pancreatic cancer cells lacking KRAS function. Nat Commun 2017;8:1090. [Crossref] [PubMed]
- Will M, Qin AC, Toy W, et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov 2014;4:334-47. [Crossref] [PubMed]
- Diaz-Flores E, Goldschmidt H, Depeille P, et al. PLC-gamma and PI3K link cytokines to ERK activation in hematopoietic cells with normal and oncogenic Kras. Sci Signal 2013;6:ra105. [Crossref] [PubMed]
- Yan C, Huo X, Wang S, et al. Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. J Hepatol 2015;63:420-8. [Crossref] [PubMed]
- Dias Carvalho P, Guimaraes CF, Cardoso AP, et al. KRAS Oncogenic Signaling Extends beyond Cancer Cells to Orchestrate the Microenvironment. Cancer Res 2018;78:7-14. [Crossref] [PubMed]
- Mollinedo F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol 2019;40:228-42. [Crossref] [PubMed]
- Phan VT, Wu X, Cheng JH, et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci U S A 2013;110:6079-84. [Crossref] [PubMed]
- Reid MA, Kong M. Dealing with hunger: Metabolic stress responses in tumors. J Carcinog 2013;12:17. [Crossref] [PubMed]
- Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011;13:132-41. [Crossref] [PubMed]
- Kimmelman AC, White E. Autophagy and Tumor Metabolism. Cell Metab 2017;25:1037-43. [Crossref] [PubMed]
- Das G, Shravage BV, Baehrecke EH. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol 2012;4:a008813. [Crossref] [PubMed]
- Chen Q, Kang J, Fu C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct Target Ther 2018;3:18. [Crossref] [PubMed]
- Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer 2017;17:528-42. [Crossref] [PubMed]
- Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014;514:628-32. [Crossref] [PubMed]
- Garcia-Navas R, Munder M, Mollinedo F. Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy 2012;8:1557-76. [Crossref] [PubMed]
- Cai Y, Arikkath J, Yang L, et al. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 2016;12:225-44. [Crossref] [PubMed]
- Gajate C, Matos-da-Silva M, Dakir EL, et al. Antitumor alkyl-lysophospholipid analog edelfosine induces apoptosis in pancreatic cancer by targeting endoplasmic reticulum. Oncogene 2012;31:2627-39. [Crossref] [PubMed]
- Chude CI, Amaravadi RK. Targeting Autophagy in Cancer: Update on Clinical Trials and Novel Inhibitors. Int J Mol Sci 2017;18:1279. [Crossref] [PubMed]
- Verbaanderd C, Maes H, Schaaf MB, et al. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 2017;11:781. [Crossref] [PubMed]
- Bryant KL, Stalnecker CA, Zeitouni D, et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med 2019;25:628-40. [Crossref] [PubMed]
- Hayes TK, Neel NF, Hu C, et al. Long-Term ERK Inhibition in KRAS-Mutant Pancreatic Cancer Is Associated with MYC Degradation and Senescence-like Growth Suppression. Cancer Cell 2016;29:75-89. [Crossref] [PubMed]
- Papademetrio DL, Lompardia SL, Simunovich T, et al. Inhibition of Survival Pathways MAPK and NF-kB Triggers Apoptosis in Pancreatic Ductal Adenocarcinoma Cells via Suppression of Autophagy. Target Oncol 2016;11:183-95. [Crossref] [PubMed]
- Lee CS, Lee LC, Yuan TL, et al. MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival. Proc Natl Acad Sci U S A 2019. [Epub ahead of print]. [PubMed]
- Melo-Lima S, Gajate C, Mollinedo F. Triggers and signaling cross-talk controlling cell death commitment. Cell Cycle 2015;14:465-6. [Crossref] [PubMed]
- Melo-Lima S, Lopes MC, Mollinedo F. ERK1/2 acts as a switch between necrotic and apoptotic cell death in ether phospholipid edelfosine-treated glioblastoma cells. Pharmacol Res 2015;95-96:2-11. [Crossref] [PubMed]
- Burgeiro A, Gajate C. Involvement of mitochondrial and B-RAF/ERK signaling pathways in berberine-induced apoptosis in human melanoma cells. Anticancer Drugs 2011;22:507-18. [Crossref] [PubMed]


