
Perioperative lymphocytopenia predicts mortality and severe complications after intestinal surgery
Introduction
Both cellular and humoral immune mechanisms play an important role in the systemic response to surgical injury and tissue healing, especially after procedures requiring visceral resections (1). Major surgery induces an activation of the hypothalamus-hypophysis-adrenal axis, followed by a spleen rapid mobilization of neutrophilic granulocytes and their massive influx in the bloodstream together with blood lymphocyte depletion. This reversible deficit of lymphocytes in the blood is caused by a redistribution of these cells into primary and secondary lymphatic organs, which happens in few hours (2). The recovery of lymphocyte count in peripheral blood takes approximately 36 to 72 hours. At present, there is no clear association, demonstrated in clinical settings, between postoperative complications and immune impairment of the patients. Nevertheless, some previous exploratory experiences are intriguing (3,4). Peripheral blood total lymphocyte count, assessed by differential automated count, has been found to be a valuable predictor of survival in patients with advanced cancer, both at baseline and after different pharmacologic treatment (5,6).
We hypothesized that major complications after abdominal surgery were associated with impaired immune response of the patients. The aim of this exploratory retrospective study is to verify whether postoperative mortality, postoperative morbidity and anastomotic leak (AL) are associated with a selected pattern of white blood cell (WBC) differential count, in patients who underwent abdominal surgery with bowel anastomosis without diverting stoma. In order to examine their real prognostic significance, we assessed the WBC differential count parameters together with major clinical variables, which are known to affect postoperative outcomes.
Methods
Inclusion and exclusion criteria
After local Ethics Committee approval (protocol 0016932/17U), clinical, pathologic and laboratory data of patients who underwent consequentially lower gastro-intestinal surgery (urgent or elective) in our General Surgery Division since June 2014 to June 2017 were retrospectively collected and analyzed. All patients received bowel resection (any intestinal segment: jejunum-ileum, colon or rectum) and anastomosis. Patients submitted to intestinal resection with concomitant diverting stoma or patients who underwent a Hartmann's procedure were excluded. In addition, patients with history of neo-adjuvant radio/chemotherapy or lymphoma/leukemia were excluded from the study.
Data collection and selection of variables
Written informed consent for data collection was obtained from each patient. Population, laboratory, intra- and post-operative data were collected by the investigators from the hospital electronic records of the General Surgery Division.
Routine demographic variables were obtained, including age at time of surgery and gender. Age-adjusted Charlson comorbidity index (ACCI) score of each patient was calculated (7). Clinical, operative, and pathological characteristics were collected, including type of resection (small intestinal, colonic or rectal resections), operative time, presence of bowel obstruction, peritonitis or intestinal ischemia, diagnosis of inflammatory bowel disease (IBD) and use of steroids. In case of colorectal cancer, tumor stage was registered. Complete blood count (CBC) at baseline (by blood samples taken within one month before surgery in elective procedures and at the time of admission in urgent surgery) and values taken on postoperative days (24–72 hours) were registered. In case of more than one postoperative value being available, the median value was calculated. Laboratory parameters included absolute neutrophil count, absolute lymphocyte count and absolute monocyte count. Overall 23 predictors of interest were selected: age, gender, ACCI, urgent operation, bowel obstruction, peritonitis, intestinal ischemia, colorectal cancer, cancer stage I–II, cancer stage III–IV, IBD, steroid therapy, operation time, small bowel resection, right hemicolectomy, left hemicolectomy, rectal resection, pre- and postoperative lymphocyte count, pre- and postoperative monocyte count and pre- and postoperative neutrophil count.
Outcomes
The primary clinical outcome was post-operative mortality; secondary outcomes were postoperative morbidity and occurrence of AL. Postoperative mortality was defined as in-hospital death occurring within 30 days after surgery. Therapeutic procedures for treatment of complications, reoperations, unplanned admissions to ICU, were registered and classified according to the Clavien-Dindo grading (8). All patients with a Clavien-Dindo grade II or lower were considered to have developed a minor complication, while those with a Clavien-Dindo grade III or higher were considered to have suffered a major complication. Deaths were excluded from the analysis of post-operative morbidity.
AL was graded according to International Study Group of Rectal Cancer (ISGRC) criteria as follows: AL requiring no active therapeutic intervention (grade A), AL requiring active therapeutic intervention but manageable without re-laparotomy (grade B) and AL requiring re-laparotomy (grade C) (9). Of interest, ISGRC proposed a grading system for AL in patients submitted to anterior resection for rectal cancer. We decided to adopt this classification system because it represents the most validated grading score in the literature and can be easily applied even when different types of intestinal anastomosis are taken in consideration. ALs were diagnosed clinically and confirmed by imaging. Because of their minor clinical significance, grade A leakages were not considered in our analysis.
Statistical analysis
Continuous variables were expressed as means ± standard deviations. Categorical variables were presented as frequencies and percentages. The outcomes of the study were expressed as dichotomous data and consequently, a logistic regression model was used for all of them. The multicollinearity of continuous variables was measured by Pearson correlation coefficients (coefficient >0.8 was considered indicative of high collinearity of two variables). Initial variable analysis was carried out with univariate logistic regression: all variables that were significant at P value <0.25 (Wald test) were considered for the multivariable model (10). The main effects model was built with a forward sequential logistic regression with selection of variables associated with a significant p value. To evaluate the relevance of potential confounders and clinically relevant interactions, likelihood ratio tests were performed between models with and without confounders and interactions (10). In the final regression model, the results were presented as odds ratio with 95% confidence interval and P value <0.05. The Hosmer-Lemeshow test was performed for assessing goodness of fit of the final model. The area under the receiving operating characteristic (ROC) curve was calculated to illustrate the ability of the multivariable logistic regression model to predict the primary outcome of the study. Statistical analysis was performed by using STATA® version 14 (StataCorp LLC, College Station, TX).
Results
During the study period (June 2014 to June 2017) 301 patients were retrospectively analyzed. The mean age of our sample was 70 years (range, 25–93 years). One hundred and sixty-five (54.8%) patients were males and ACCI mean value was 5 (range, 0–14 years). Colorectal cancer was the predominant indication for surgery (59.8%), with 86 (28.6%) patients having an advanced disease at presentation (stage III–IV). Urgent surgery was performed in 177 (58.8%) cases. Eighty-nine (29.6%) patients had bowel obstruction at time of surgery, while 59 (19.6%) patients had local or generalized peritonitis due to intestinal perforation. Left hemicolectomy was the most frequent procedure (100 cases, 33.2%). Mean operation time was 2.5 hours (range, 32 minutes to 9.25 hours) with 150 (49.8%) procedures lasting longer than 150 minutes. Twelve (4.0%) patients were taking steroids at the time of surgery, while 13 (4.3%) people had a history of IBD. Clinical and operative characteristics are listed in Table 1.
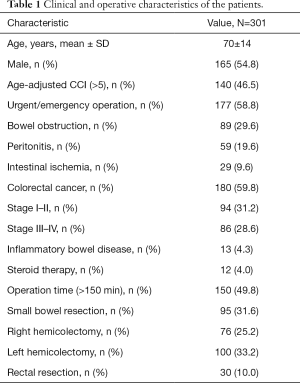
Full table
Postoperative mortality
Twelve people died within 30 days after surgical operation, resulting in an in-hospital postoperative mortality rate of 4%. Univariate analysis showed 11 variables associated with higher risk of postoperative mortality: age (P=0.008), ACCI (P<0.001), urgent/emergent operation (P=0.094), bowel obstruction (P=0.125), right hemicolectomy (P=0.217), AL (grade B,C) (P=0.004), preoperative lymphocyte count (P=0.100), preoperative neutrophil count (P=0.213), preoperative monocyte count (P=0.012), postoperative neutrophil count (P<0.001), and postoperative monocyte count (P=0.098).
ACCI (OR =1.69; 95% CI, 1.20–2.36; P=0.002), AL (grade B,C) (OR =17.22; 95% CI, 2.60–113.97; P=0.003), low preoperative lymphocyte count (OR =0.11; 95% CI, 0.02–0.72; P=0.021), high preoperative monocyte count (OR =5.64; 95% CI, 1.13–28.03; P=0.034), and high postoperative neutrophil count (OR =1.32; 95% CI, 1.07–1.62; P=0.008) were significantly associated with 30-days mortality in the multivariate analysis (Table 2). The models that included the possible confounders present in the literature (age, urgent/emergent surgery, malignant tumor) were not more informative than the final model at likelihood ratio test. No significant interactions were present in the final model. The P value of the Hosmer-Lemeshow (goodness-of-fit) test was 0.657. The area under ROC curve of the multivariate regression model predicting postoperative mortality was 0.938 (Figure 1).
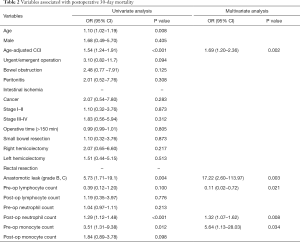
Full table

Postoperative morbidity
Postoperative morbidity was observed in 124 (41%) patients. Minor complications according to Clavien-Dindo classification occurred in 80 (27%) patients: 20 (7%) patients with grade I and 60 (20%) patients with grade II complications respectively. Major complications, graded as Clavien-Dindo III and IV, occurred in 44 (15%) patients, distributed as follows: 12 (4%) patients with grade III-a, 25 (8%) patients with grade III-b and 7 (2%) patients with grade IV complications. Univariate analysis showed that age (P=0.219), male sex (P=0.029), operative time (P=0.097), small bowel resection (P=0.027), right hemicolectomy (P=0.070), rectal resection (P=0.019), pre-operative low lymphocyte count (P=0.019), postoperative low lymphocyte count (P=0.043) and postoperative high monocyte count (P=0.112) were statistically associated with major postoperative complications. In the multivariate analysis rectal resection (OR =2.83; 95% CI, 1.16–6.89; P=0.021) and low pre-operative lymphocyte count (OR =0.50; 95% CI, 0.27–0.93; P=0.029) were found to be independent variables associated with the development of grade III-IV complications (Table 3). The models with the possible confounders (age, urgent/emergent surgery, malignant tumor) were not more informative than the final model at likelihood ratio test. No significant interactions were present in the final model. The P value of the Hosmer-Lemeshow (goodness-of-fit) test was 0.275.
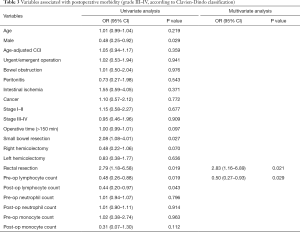
Full table
Anastomotic leak
Anastomotic leaks occurred in 37 (12%) patients (grade B and C). Of these 22 cases needed re-laparotomy (grade C), while 15 patients were treated by percutaneous drainage and antibiotics (grade B). Univariate analysis showed 12 variables associated with AL: male gender (P=0.249), ACCI (P=0.226), urgent/emergent operation (P=0.214), cancer (P=0.085), cancer stage I–II (P=0.096), operative time (>150 min) (P=0.027), small bowel resection (P=0.183), rectal resection (P=0.015), postoperative lymphocyte count (P=0.009), preoperative neutrophil count (P=0.039), preoperative monocyte count (P=0.110) and postoperative monocyte count (P=0.076). In the multivariate analysis low postoperative lymphocyte count (OR =0.31; 95% CI, 0.13–0.75; P=0.010) was significantly associated with developing a leak. It must be noted that rectal resection (OR =2.59; 95% CI, 0.99–6.76; P=0.051) was associated with a high risk of AL, with a borderline P value, very close to the conventional significance level (P=0.05), although odds ratio confidence interval contained the unit (Table 4). The models that included the possible confounders (age, urgent/emergent surgery, malignant tumor) were not more informative than the final model at likelihood ratio test. The P value of the Hosmer-Lemeshow (goodness-of-fit) test was 0.528.
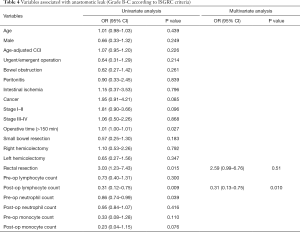
Full table
Discussion
In this study, we investigated the relevance of WBC differential count patterns as independent risk factors of postoperative morbidity and mortality in patients who underwent intestinal resection and anastomosis, together with known clinical risk factors. We found that preoperative lymphocytopenia was independently associated with postoperative mortality and morbidity, while a low postoperative lymphocyte count was predictive of AL.
Many clinical variables seem to be involved in postoperative mortality after intestinal resection: advanced age, male sex, urgent surgery, cancer, total colectomy and AL (11-15). Even if large retrospective studies have assessed the postoperative mortality of colorectal surgery patients, nevertheless there is no clear consensus regarding major risk factors (11-15).
The possible confounders in the relationship between mortality/morbidity and WBC count parameters were tested in our model of multivariable regression: age, urgent surgery and malignant tumor. Moreover, we employed a restriction criterion for such conditions known to be cause of lymphocytopenia (neo-adjuvant radiotherapy or chemotherapy).
Differently from the majority of previous studies, urgent/emergent surgery and age were associated with mortality at univariate analysis, but not at multivariate analysis (11-13). On the other side, we observed that ACCI and AL were significantly associated with in-hospital 30-day death, in accordance to two prior studies (14,15). The ACCI summarizes information about the presence of a number of medical conditions in order to create a single measure of comorbidity. It has showed a good ability to discriminate the outcomes, with a special regard to hospital mortality (16).
In the same way, a large amount of studies has been devoted to the study of AL and other postoperative complications after intestinal resection. In these studies, several clinical and biologic findings have been found to be associated with a higher risk of AL, including male sex, emergency surgery, rectal resection, operative time, steroid therapy, nonsteroidal anti-inflammatory drugs and obesity, showing a large variability in the results (17-20). In our analysis, rectal resection was significantly associated with both AL and postoperative morbidity.
In our retrospective study, we found that the introduction of the differential WBC count in the statistical analysis was able to identify a sub-set of patients who developed severe complications and death after surgery. Interestingly, fluctuations in WBC differential count showed the same or even a stronger value to predict poor outcomes compared to the traditional high-risk factors associated with mortality, morbidity and AL after intestinal surgery. In fact, patients showed different outcomes according to their immune status at baseline and their immune response to surgical trauma. Patients who had a normal immune pattern, represented by regular lymphocyte and monocyte count at baseline, postoperative recovery of lymphocyte count and usual increase of neutrophils at days 1 to 3, frequently had an uneventful postoperative course or developed mild complications (grade I–II, Clavien-Dindo classification). Differently, patients showing a low lymphocyte count and a high monocyte count at baseline, with a higher increase of neutrophils at days 1 to 3 were at higher risk of death at 30 days. Moreover, patients with low lymphocyte count at baseline frequently developed severe complications (grade III–IV, Clavien-Dindo classification), and those with postoperative lack of lymphocyte recovery in many cases developed AL (grade B-C).
The immune function can be measured in two different settings: at baseline, in a static condition, and after surgery, during a dynamic response (1). After an immediate decrease in circulating lymphocytes occurring within a few hours from surgical trauma, the recovery of the lymphocyte count in peripheral blood, together with the increase in neutrophils, begins approximately 24 hours after surgery (2,21). Thus, we chose the 24-hour period after surgery as the first time point to measure postoperative differential count, until day 3 (72 hours after surgery). After three days, in fact, surgical complications usually become clinically evident. Interestingly, patients with preoperative lymphopenia had a significantly higher incidence of complications and a higher death rate compared with those who had a normal lymphocyte level at admission.
Concerning immune response after surgery, our results support previous observations in trauma and septic patients where failure to normalize lymphopenia was associated with an increased mortality (22,23). In a recent study by Vulliamy et al., persistent lymphopenia was an independent predictor of increased mortality in critically ill emergency surgical patients (3). This result reflects the concept that lymphocyte recovery in the first post-operative days could play an important role in the mechanisms of tissue repair and a primary role in patient healing. Thus, the evaluation of WBC differential count, especially lymphocyte count at baseline and after surgery, may allow surgeons to identify patients at high risk of death and postoperative complications in order to make the best individualized care decisions, both in elective and urgent settings. For example, the creation of loop ileostomy in patients’ candidate for elective rectal resections and found with low preoperative lymphocyte count should be encouraged in order to reduce the risk of AL and severe morbidity. Moreover, considering the management of patients presenting with colorectal obstruction with impaired WBC count, the placement of self-expanding stents and delayed surgery should be considered a valuable alternative approach in order to reduce the incidence of postoperative complications and stoma rate.
Previous studies on neutrophil to lymphocyte (N/L) ratio explored the association between high N/L ratio and postoperative complications (24,25). A high postoperative ratio was found to be an easy calculable preoperative measure to possibly predict the outcome after abdominal and colorectal surgery. In our patients, a high postoperative neutrophil count was significantly associated with death, while lymphocyte count was predictive as a low value preoperatively. Indeed, the N/L ratio may confound the interpretation of different immune patterns of patient response to surgical trauma at different time points, thus its real value as a predictive factor of surgical outcome should be considered with caution. Furthermore, the introduction of N/L ratio in the regression model could lead to problems of collinearity in the statistical analysis.
As we reported above, a high preoperative monocyte count was found to be related to 30-days postoperative mortality at multivariate analysis. Despite many studies have identified a relationship between the peripheral blood monocyte count and the survival rate of colorectal cancer patients, there is a lack of evidence about the role of high preoperative monocyte count as a predictive marker of postoperative mortality in subjects submitted to intestinal surgery (26,27).
Our study results support the relevance of the WBC differential count as a crucial player and marker of postoperative outcomes in patients submitted to major intestinal surgery. In our patients, lymphocyte, monocyte and neutrophil count resulted to be more relevant than other known risk factors for complications such as age, male gender, urgency setting, operative time or malignancy (11-15,17-20). Accordingly, our final multivariate model is characterized by a considerable area under the ROC curve (0.93) determining a set of variables which are highly correlated with postoperative mortality (Figure 1). Nevertheless, it is necessary to underline some limitations of the present study. This is a retrospective collection of data and, although it represents the routine clinical practice, this makes our research less generalizable than a prospective one. All surgical interventions were performed both in elective and urgent setting and this could be considered a potential weakness of the study. However, as emerged from our results, urgent/emergent operation was not found to be significantly associated with mortality, death or AL in the multivariate analysis, thus its ability to influence the outcomes in our population should be considered with cautious. Moreover, not all the patients had continuously laboratory draws in the first, second and third postoperative days. Our in-hospital death rate was 4%, and this value does not differ significantly from the largest nationwide series for colorectal resections, reporting a postoperative mortality range from 2% to 8% (11-13). Although the comorbidity rate (41%) was in line with those reported in the literature (12,25,28,29), the incidence of AL was quite high compared to other studies (18,19). Some reasons could explain this higher rate of AL. First, half of the procedures were performed in an emergency setting and without stoma diversion (58.8%). Second, a large percentage of patients (47%) had an ACCI score greater than 5, which reflects a population at higher risk for surgical complications both in terms of ageing and of severe comorbidities.
Conclusions
Our study represents a common sample of daily surgical care and reflects demographic and public health resource challenges in the Western world. It refocuses the crucial role of immune status and immune response in healing processes in surgical patients. Furthermore, these results suggest that immune monitoring by a widely available, easily readable, and low-cost test, such as automated differential blood cell count may early add critical information on surgical patients risks and, once operated, even on clinical course. Patients having low lymphocyte counts were found to have a higher risk of developing major surgical complications and death, while postoperative lack of lymphocyte recovery was associated with a higher AL rate. In a short-term view, once validated, the evaluation of differential WBC count patterns may improve a tailored planning and monitoring of surgical strategy. In a future view, a therapeutic modification (30) of host immune patterns in response to major surgery may eventually represent an effective strategy to improve surgical outcomes.
Acknowledgments
This study is supported by the Department of Surgery, University of Milan-Bicocca - Istituti Clinici Zucchi - Monza – Italy. All authors are in debt with head of Surgical Department at Ospedale A. Manzoni – Lecco (Italy), M. Costa, FRCSEd, for his precious teaching in daily duties and would like to thank Prof. Fausto Galli, Assistant Professor of Statistics at the University of Salerno, for assistance with statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Local Research Ethics Committee, the Socio-Sanitary Territorial Unit Of Monza (No. 0016932/17U). Informed consent for participation in the study was obtained either directly, or from a guardian of each patient.
References
- Lundy J, Ford CM. Surgery, trauma and immune suppression. Evolving the mechanism. Ann Surg 1983;197:434-8. [Crossref] [PubMed]
- Toft P, Tønnesen E, Helbo-Hansen HS, et al. Redistribution of granulocytes in patients after major surgical stress. APMIS 1994;102:43-8. [Crossref] [PubMed]
- Vulliamy PE, Perkins ZB, Brohi K, et al. Persistent lymphopenia is an independent predictor of mortality in critically ill emergency general surgical patients. Eur J Trauma Emerg Surg 2016;42:755-60. [Crossref] [PubMed]
- Brivio F, Fumagalli L, Lissoni P, et al. Pre-operative immunoprophylaxis with interleukin-2 may improve prognosis in radical surgery for colorectal cancer stage B-C. Anticancer Res 2006;26:599-603. [PubMed]
- Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012;10:33. [Crossref] [PubMed]
- Fumagalli LA, Vinke J, Hoff W, et al. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother 2003;26:394-402. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery 2010;147:339-51. [Crossref] [PubMed]
- Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 2013 Wiley 3rd edition.
- Tekkis PP, Poloniecki JD, Thompson MR, et al. Operative mortality in colorectal cancer: prospective national study. BMJ 2003;327:1196-201. [Crossref] [PubMed]
- Alves A, Panis Y, Mathieu P, et al. Association Française de Chirurgie. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 2005;140:278-83. [Crossref] [PubMed]
- Masoomi H, Kang CY, Chen A, et al. Predictive factors of in-hospital mortality in colon and rectal surgery. J Am Coll Surg 2012;215:255-61. [Crossref] [PubMed]
- Bakker IS, Grossmann I, Henneman D, et al. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424-32. [Crossref] [PubMed]
- Morris EJ, Taylor EF, Thomas JD, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011;60:806-13. [Crossref] [PubMed]
- Poses RM, McClish DK, Smith WR, et al. Prediction of survival of critically ill patients by admission comorbidity. J Clin Epidemiol 1996;49:743-47. [Crossref] [PubMed]
- Suding P, Jensen E, Abramson MA, et al. Definitive risk factors for anastomotic leaks in elective open colorectal resection. Arch Surg 2008;143:907-11. [Crossref] [PubMed]
- Nikolian VC, Kamdar NS, Regenbogen SE, et al. Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surgery 2017;161:1619-27. [Crossref] [PubMed]
- Lipska MA, Bissett IP, Parry BR, et al. Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ J Surg 2006;76:579-85. [Crossref] [PubMed]
- Bakker N, Deelder JD, Richir MC, et al. Risk of anastomotic leakage with nonsteroidal anti-inflammatory drugs within an enhanced recovery program. J Gastrointest Surg 2016;20:776-82. [Crossref] [PubMed]
- Hamid J, Bancewicz J, Brown R, et al. The significance of changes in blood lymphocyte population following surgical operations. Clin Exp Immunol 1984;56:49-57. [PubMed]
- Heffernan DS, Monaghan SF, Thakkar RK, et al. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care 2012;16:R12. [Crossref] [PubMed]
- Drewry AM, Samra N, Skrupky LP, et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014;42:383-91. [Crossref] [PubMed]
- Vaughan-Shaw PG, Rees JR, King AT. Neutrophil lymphocyte ratio in outcome prediction after emergency abdominal surgery in the elderly. Int J Surg 2012;10:157-62. [Crossref] [PubMed]
- Josse JM, Cleghorn MC, Ramji KM, et al. The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Colorectal Dis 2016;18:O236-42. [Crossref] [PubMed]
- Hu S, Zou Z, Li H, et al. The Preoperative Peripheral Blood Monocyte Count Is Associated with Liver Metastasis and Overall Survival in Colorectal Cancer Patients. PLoS One 2016;11:e0157486. [Crossref] [PubMed]
- Abe S, Kawai K, Nozawa H, et al. LMR predicts outcome in patients after preoperative chemoradiotherapy for stage II-III rectal cancer. J Surg Res 2018;222:122-31. [Crossref] [PubMed]
- Duraes LC, Stocchi L, Steele SR, et al. The Relationship Between Clavien-Dindo Morbidity Classification and Oncologic Outcomes After Colorectal Cancer Resection. Ann Surg Oncol 2018;25:188-96. [Crossref] [PubMed]
- McSorley ST, Ramanathan ML, Horgan PG, et al. Postoperative C-reactive protein measurement predicts the severity of complications following surgery for colorectal cancer. Int J Colorectal Dis 2015;30:913-7. [Crossref] [PubMed]
- Brivio F, Lissoni P, Alderi G, et al. Preoperative interleukin-2 subcutaneous immunotherapy may prolong the survival time in advanced colorectal cancer patients. Oncology 1996;53:263-8. [Crossref] [PubMed]


