
Quality of life in patients with bronchiectasis: a 2-year longitudinal study
Introduction
Bronchiectasis is a chronic respiratory disorder characterized by daily productive cough, dyspnea, and fatigue. Acute exacerbations of airway infection are common, typically treated with antibiotics, and often result in hospitalization (1). Since patients with bronchiectasis often have a poor health-related quality of life (HRQoL), increased health care utilization, and poor outcomes, there has been an increased interest in measuring HRQoL using validated, condition-specific instruments to evaluate whether specialized care and new interventions can improve daily functioning (2). The St. George’s Respiratory Questionnaire [designed for chronic obstructive pulmonary disease (COPD)] and Leicester Cough Questionnaire (designed for cough) have been used to evaluate the HRQoL in patients with bronchiectasis (3,4). Since these instruments are neither bronchiectasis-specific, nor do they meet the Food and Drug Administration (FDA) guidance on patient-reported outcomes (PROs), a new questionnaire was developed, called the Quality of Life Questionnaire-Bronchiectasis (QOL-B) by Quittner et al., which overcomes both issues. It assesses symptoms, physical, emotional functioning and treatment burden. It is comprised of thirty-six items on eight scales (physical functioning, role functioning, vitality, emotional functioning, social functioning, treatment burden, health perceptions, and respiratory symptoms) (5). Few studies to date have examined longitudinal outcomes on the QOL-B in relation to specialized care or specific, prescribed treatment. A study by McCullough et al. in which the QOL-B questionnaire was used at the time of study enrollment and after 12 months examined whether adherence to inhaled antibiotics, other respiratory medicines, and airway clearance led to improved patient outcomes (6). No studies have been published in which serial QOL-B questionnaires have been administered to patients over more than one year. Because bronchiectasis is a chronic condition, we were interested in measuring HRQoL in bronchiectasis patients over a longer period of time. We were also interested in assessing changes in QOL-B scores after the initial visit to the University of Connecticut Center for Bronchiectasis Care, to determine if improvements in HRQoL could be detected after initial treatment at a center dedicated to the care of patients with bronchiectasis
Methods
QoL-B instrument
The version of the QOL-B used was QOL-B V3.1. The domains assessed in QOL-B by Quittner et al. include physical functioning, role functioning, vitality, emotional functioning, social functioning, treatment burden, health perceptions, and respiratory symptoms. For each scale, scores are standardized on a 0- to 100-point scale; higher scores indicate better HRQoL (including treatment burden); no total score is calculated since functioning can vary greatly from one domain to another. A multi-center study of this instrument demonstrated good reliability, internal consistency, test-retest, and convergent validity with other respiratory HRQoL measures (7).
Study design
We performed a retrospective analysis of prospectively obtained QOL-B data from adult patients with non-cystic fibrosis bronchiectasis. Patients were enrolled between 2008 and 2011 during their initial visit to the University of Connecticut Center for Bronchiectasis Care. While an attempt was made to enroll consecutive patients, in some cases, potentially eligible patients were not enrolled due to time limitations related to the delivery of clinical care in a busy outpatient practice. The University of Connecticut institutional review board approved the study and waived the requirement for informed consent, because the QOL-B was administered as part of standard of care at the Bronchiectasis Center. The QOL-B was administered yearly during routine outpatient appointments, until the baseline and two yearly follow-up questionnaires were obtained. If the patient was in the midst of an exacerbation, or had recently had an exacerbation, the QOL-B administration was postponed until the patient had returned to their baseline. The responses from the 36-item questionnaire were scored on the eight domains and a scaled score was calculated for each.
Participants and setting
Patients with a confirmed diagnosis of non-cystic fibrosis bronchiectasis by computed tomography scan, who were at least eighteen years old, and who were new patients to the University of Connecticut Center for Bronchiectasis Care at the time their HRQoL was first measured, were enrolled in this study. Patients who had participated in other studies using the QOL-B and those who did not speak English were excluded from the study. The University of Connecticut Center for Bronchiectasis Care provides multi-disciplinary care for patients with bronchiectasis. All patients are provided disease specific education and symptomatic patients are started on an airway clearance regimen with either a positive expiratory pressure device or high frequency chest wall oscillation vest with or without hypertonic saline nebulization. Depending upon severity of the disease and symptoms, patients may be treated with inhaled antibiotics and/or chronic low dose macrolide therapy. Underlying etiologies such as nontuberculous mycobacterium infection or humoral immunodeficiency are treated, as indicated.
Data elements and statistical analysis
Baseline characteristics included: age, gender, ethnicity, body mass index (BMI), current and/or previous smoker, forced expiratory volume (FEV1) (L), FEV1% predicted, forced vital capacity (FVC) (L), FVC % predicted, and time between QOL-B 1 and QOL-B 3. Other characteristics analyzed were smoking status and pulmonary function tests, including FEV1, FVC, and FEV1/FVC. Mean differences were compared using student paired t-tests. Analysis was performed using SPSS software, version 12.0 (SPSS Inc., Chicago, IL, USA). Statistical significance between QOL-B 1, QOL-B 2, and QOL-B 3 was calculated with a two-tailed P value of significance of less than 0.05.
Results
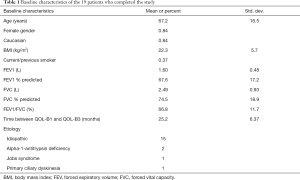
Twenty-six patients completed an initial QOL-B, however seven of these patients did not complete all three administrations and thus, were excluded from the primary analyses. Among the nineteen patients who completed all assessments, 16 (84%) percent were female and mean age was 67.2±16.5 years old. The mean time between administration of the first QOL-B and the second QOL-B was 12.8±4.9 months, with 25.2±6.37 months between the first and third QOL-B. Seven (37%) patients were smokers. The mean FEV1/FVC ratio was 66.79%±11.72%. Baseline demographic and other patient characteristics are shown in Table 1. The majority of patients enrolled in this study had idiopathic bronchiectasis. Other causes included alpha-1-antitrypsin deficiency, Jobs syndrome, and primary ciliary dyksinesia.
Full table
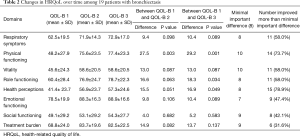
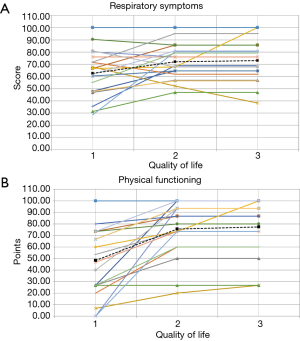
Table 2 demonstrates the HRQoL results among the 19 patients included in the study, as well as the validated minimally important difference (MID) for each of the 8 domains (8). Overall, between six and fifteen patients improved by more than the MID for each of the 8 domains. An average of 2.5 patients worsened by the MID for all categories of the QOL-B combined. Statistically significant improvements in mean QOL-B scores were observed for 3 of the 8 domains from baseline to the third assessment. As can be seen, for most of the domains, most of the improvement was reported by patients between the 1st QOL-B assessment and the 2nd administration. Importantly, QOL-B scores remained relatively stable between the second and third measurement. In Figure 1A,B, patient level data is shown for two of the more important domains: Respiratory Symptoms and Physical Functioning. We reviewed the 7 patients that were excluded because they did not complete all three assessments, reasons included: patients moving away [2]; patient death [1]; failure to complete subsequent QOL-B, although on follow-up patients were doing well [3]; and a patient was lost to follow-up for an undocumented reason [1]. Of the 7 patients who did not complete three assessments, 4 of these patients completed QOL-B1 and QOL-B2. Table 3 shows the QOL-B scores for these four patients. As can be seen, their results are similar to those who completed all 3 QOL-Bs.
Full table

Full table
Discussion
Bronchiectasis is generally an incurable disease, with significant impact on patients’ quality of life, yet little is known about changes in HRQoL over time. Understanding the long-term effects of this disease on daily functioning and symptoms is important to better understand the natural history of the disease, and to evaluate the efficacy of long-term treatments. To our knowledge, not only is this the first longitudinal study to measure HRQoL in bronchiectasis patients over more than a one-year period, but it also demonstrated that care for patients in specialized bronchiectasis centers may have an important effect on quality of life outcomes for these patients.
In this longitudinal study of 19 patients with non-CF bronchiectasis, we found statistically significant mean improvements in three domains on the QOL-B, two years after a baseline measurement (Physical Functioning, Role Functioning, and Health Perceptions), with non-significant trends towards improvement in the other 5 domains. For each of the 8 domains, between 6 and 15 patients improved above the established MID. The improvements were generally maintained at 2 years after the baseline assessment. The MID, which provides a measure of the smallest change in the outcome that patients perceive as important, and thus, clinically meaningful, was achieved in seven out of the eight domains.
Although prior studies have used the QOL-B to measure HRQoL longitudinally, none have been for greater than 1 year. In 2008, Courtney et al. published their work using a different questionnaire, Chronic Respiratory Disease Questionnaire, to measure changes in HRQoL at the beginning and after resolution of a bronchiectasis exacerbation. They also studied how changes in HRQoL were related to changes in airway physiology, airway inflammation, and systemic inflammation markers. They administered the questionnaire at day 1, 2 weeks, and 4 weeks after intravenous antibiotics and found a significant improvement in the domains of dyspnea, emotional, and mastery (9). Other studies have measured HRQoL in children with non-CF bronchiectasis to assess for risk factors, such as age at diagnosis, follow-up period, pulmonary function test, high resolution computed tomography score, and socioeconomic status. Follow-up with a QOL questionnaire, specifically the generic short-form-36 was within weeks of assessment (10). More recently, Barker et al. performed a phase 3 clinical trial with the aim to assess the safety and efficacy of inhaled aztreonam in patients with non-cystic fibrosis bronchiectasis and gram-negative bacterial colonization using the QOL-B Respiratory Symptom scores (QOL-B-RSS). The QOL-B-RSS was administered 4 weeks after the first treatment course and at 12 weeks, immediately after the second treatment course. This study found no significant clinical benefits for patients with non-cystic fibrosis bronchiectasis, suggesting the continued need for further research (11). Soon after McCullough, et al. performed a study to assess the adherence to inhaled antibiotics, other respiratory medicines and airway clearance to determine the association between adherence to these treatments and health outcomes such as pulmonary exacerbations, lung function, and QOL-B scores in bronchiectasis after 12 months. The QOL-B was administered at baseline and after 12 months and demonstrated that adherence to airway clearance was associated with improvements in physical functioning, but worse scores on treatment burden and respiratory symptoms (6). These results were only found for adherence to airway clearance but not to inhaled antibiotics or other respiratory medications. No other studies have examined serial QOL-B scores collected over more than 1 year.
Despite showing improvement in patient reports of HRQoL, there were limitations encountered in this study. The primary limitation was the small sample size enrolled in this study, raising the risk of a type 2 statistical error in the domains for which a statistically significant improvement was not found. Seven patients did not complete the 3rd QOL-B assessment, which raises concerns about selection bias toward reporting positive results. However, four of these seven patients demonstrated improvements in their QOL-B at the first follow up assessment, with a pattern that was similar to the large cohort. Finally, we only followed patients for two years and do not know how they fared subsequent to completion of the study.
It is also important to note that this study population was evaluated and treated in a specialized center for patients with bronchiectasis. It is not known if such favorable results would be seen in patients treated by community pulmonologists or internists. Nonetheless, these results demonstrate that bronchiectasis is not necessarily a disease in which patients are destined to suffer an inexorable decline with worsening cough, sputum production, dyspnea and other symptoms, as has been suggested by other experts (12).
Conclusions
We demonstrated that many patients treated at a center dedicated to the care of patients with bronchiectasis reported improvements in their HRQoL, which was maintained over 2 years after the baseline visit.
Acknowledgments
Gilead Sciences provided support to AL Quittner to develop the QOL-B.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Research ethics and patient consent: UCONN HEALTH Institutional Review Board Human Subjects Protection Office IE-11-152-3. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- King PT, Holdsworth SR, Freezer NJ, et al. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006;100:2183-9. [Crossref] [PubMed]
- Amalakuhan B, Maselli DJ, Martinez-Garcia MA. Update in Bronchiectasis 2014. Am J Respir Crit Care Med 2015;192:1155-61. [Crossref] [PubMed]
- Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009;34:125-31. [Crossref] [PubMed]
- Wilson CB, Jones PW, O'Leary CJ, et al. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997;156:536-41. [Crossref] [PubMed]
- Quittner AL, Cruz I, Marciel KK, et al. Development and Cognitive Testing of the Quality of Life Questionnaire for Bronchiectasis (QOL-B). Am J Respir Crit Care Med 2009;179:A4310.
- McCullough AR, Tunney MM, Quittner AL, et al. Treatment adherence and health outcomes in patients with bronchiectasis. BMC Pulm Med 2014;14:107. [Crossref] [PubMed]
- Quittner AL, Marciel KK, Salathe MA, et al. A preliminary quality of life questionnaire-bronchiectasis: a patient-reported outcome measure for bronchiectasis. Chest 2014;146:437-48. [Crossref] [PubMed]
- Johnston BC, Ebrahim S, Carrasco-Labra A, et al. Minimally important difference estimates and methods: a protocol. BMJ Open 2015;5:e007953. [Crossref] [PubMed]
- Courtney JM, Kelly MG, Watt A, et al. Quality of life and inflammation in exacerbations of bronchiectasis. Chron Respir Dis 2008;5:161-8. [Crossref] [PubMed]
- Gokdemir Y, Hamzah A, Erdem E, et al. Quality of life in children with non-cystic-fibrosis bronchiectasis. Respiration 2014;88:46-51. [Crossref] [PubMed]
- Barker AF, O'Donnell AE, Flume P, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014;2:738-49. [Crossref] [PubMed]
- ten Hacken NH, Wijkstra PJ, Kerstjens HA. Treatment of bronchiectasis in adults. BMJ 2007;335:1089-93. [Crossref] [PubMed]


