
Bleeding in patients with atrial fibrillation treated with combined antiplatelet and anticoagulant therapy: time to turn the corner
Anticoagulation is the mainstay for stroke prevention in patients with atrial fibrillation (AF). Many patients with AF also have a concomitant indication for either single or dual antiplatelet (aspirin plus a P2Y12 inhibitor) therapy. For instance, about 1 in 3 patients with AF have coexistent coronary artery disease, and about 1 in 5 undergo percutaneous coronary intervention (PCI) (1). Although anticoagulant therapy is more effective than either single or dual antiplatelet therapy (DAPT) for stroke prevention in AF, guidelines recommend DAPT over vitamin K antagonist (VKA) for the prevention of coronary stent thrombosis. Such quandary prompted the addition of DAPT to an anticoagulant, also known as triple therapy, with the intent of preventing both coronary ischemic and cardioembolic events (2).
Data from randomized trials clearly demonstrate that bleeding is increased with combined antithrombotic regimens. Thus, compared with aspirin, the risk of major bleeding is 1.4-fold higher with DAPT with aspirin plus clopidogrel (3); and it is higher with DAPT containing a more potent P2Y12 inhibitor such as prasugrel or ticagrelor (4,5). Adding warfarin to aspirin results in a two-fold higher risk of bleeding compared with aspirin alone (6). Likewise, data from the pivotal trials of direct oral anticoagulants (DOACs) in stroke prevention in AF (SPAF) show that compared with DOAC alone, the risk of bleeding almost doubles when aspirin is used in conjunction with DOACs (7-9). The highest risk of bleeding occurs in patients with AF treated with triple therapy (10). Thus, in the WOEST trial (What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting), 31.7% of patients with AF treated with triple therapy experienced a clinically relevant bleeding, and 5.6% suffered a major bleeding within one year of PCI (10). Consequently, bleeding is problematic with triple therapy.
There is a concern that the risk of bleeding could be even higher outside of randomized controlled trials (RCTs), but until recently, robust estimates for the rate of major bleeding with various combinations in clinical practice were scarce. In a recent nationwide cohort study of over 270,000 Danish patients aged ≥50 years, with a new diagnosis of AF between 1995 and 2015, van Rein and colleagues now provide precise estimates for major bleeding rates with various antithrombotic regimens, along with their relative risks as compared with VKA monotherapy. They also provide up-to-date estimates for combination therapies with DOACs and for high risk patients who are often under-represented in RCTs (11).
As illustrated in Figure 1, the incidence rate of major bleeding was 2.3 per 100 patient-years (PYs) in those receiving VKA monotherapy, the reference group for comparison. Like the RCT data, bleeding was accentuated in AF patients receiving anticoagulation when antiplatelet agents are added and was greatest with triple therapy. By contrast, the rates of bleeding in this cohort study were almost doubled those reported in RCTs. Thus, the rates of major bleeding reached 10.4 per 100 PYs for VKA triple therapy and 8.8 per 100 PYs for DOAC triple therapy. With the use of prasugrel or ticagrelor in triple therapy, the rates of major bleeding were substantially higher and ranged from 11.8 to 24.1 per 100 PYs. Consequently, the rates of major bleeding in AF patients treated with any triple therapy are unacceptably high in clinical practice.
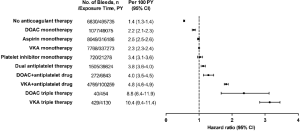
In addition, AF patients with age ≥90 years, a history of major bleeding, or CHA2DS2-VASc score >6 had the highest rates of major bleeding with any antithrombotic regimens, and rates as high as 25.1 per 100 PYs were reported in those receiving triple therapy. An increase in bleeding risk with advanced age or with a previous major bleeding episode is well established and that observed in patients with higher CHA2DS2-VASc score is also not surprising since some components of the score, e.g., age, hypertension, diabetes mellitus, and a history of ischemic stroke, are shared risk factors for bleeding (12).
The strengths of this nationwide cohort study include the large source of patient-level data, and the ability to generate precise estimates for most subgroups. Because of its observational design, the main limitations include unmeasured confounding when comparing groups. In addition, the reported rates of major bleeding may have been systematically underestimated because using ICD-10 code to identify major bleeding restricts bleeding events to those resulting in hospitalization or death only; and using prescription records for measuring drug exposure dilutes event rates because non-adherence to antithrombotic drugs is unaccounted (11). Finally, it is unfortunate that no direct analyses were presented to provide insight on the trade-off between bleeding and ischemic events. Nonetheless, the study by van Rein et al. provides highly relevant data for clinical practice, and highlights the need to address the high bleeding rates associated with triple therapy in AF patients.
Why is bleeding relevant and what can be done to address it? There is a widely held perception that stroke and myocardial infarction result in serious and permanent sequelae whereas bleeding does not (13). Emerging data, however, show that bleeding also leads to serious adverse consequences. Notwithstanding the short-term consequences (such as fatal bleeding), several studies have highlighted that patients who bleed on antithrombotic therapy have at least 5-fold higher rates of thromboembolism and mortality in the longer term (14,15). It is plausible that major bleeding could contribute to long-term adverse effects, including non-hemorrhagic vascular events or deaths, by a variety of mechanisms such as discontinuation of anticoagulant therapy, non-adherence to treatment, transfusion associated adverse effects, and reactive hypercoagulability (14-16). If so, the findings from van Rein et al. are even more concerning because the high rates of major bleeding in clinical practice, particularly in patients taking triple therapy, could have important adverse implications on the risk of subsequent thromboembolic complications or mortality. Highlighting the clinical importance of preventing bleeding is the observation that the mortality benefit with DOACs (vs. VKA) in the pivotal phase III SPAF trials was linked to a reduction in bleeding rather than in ischemic events (17). Consequently, strategies to minimize or avoid bleeding are warranted to improve the net clinical benefit of antithrombotic therapy in AF patients with a concomitant indication for antiplatelet therapy.
Two approaches to reduce bleeding have recently been evaluated. These include (I) curtailing the use of DAPT, and (II) shifting from VKA to DOAC-based therapy. Data from recent trials evaluating triple with dual antithrombotic therapy in AF patients undergoing PCI show that a strategy that limits the use of DAPT results in a 42% reduction in major bleeding (18). Similarly, the shift from VKA to DOAC-based therapy is associated with a likely 30% reduction in major bleeding in AF patients undergoing PCI, consistent with the superior safety of the DOACs as reported in the SPAF trials (18,19). In a pooled analysis that included 10,026 AF patients undergoing PCI, the combination of these two approaches i.e., the switch from VKA to DOAC and the use of single antiplatelet therapy rather than DAPT, significantly reduced major bleeding and intracranial hemorrhage (ICH) by 51% and 74%, respectively. Of relevance, the rates of major adverse cardiovascular events (odds ratio 1.02; 95% CI, 0.71–1.97) were similar but the confidence interval for the estimate was wide (18). Consequently, with the exception of those at the highest risk of coronary ischemia, recent guidelines now recommend dual antithrombotic therapy over triple therapy for AF patients undergoing PCI (20-22).
Because AF patients with age ≥90 years, a history of major bleeding, or CHA2DS2-VASc score >6, have the highest risk of bleeding, they stand to benefit the most from adopting these recommendations but the impact on efficacy remains to be defined in this population. Physicians can also help reduce bleeding by focusing efforts on assessing and addressing modifiable risk factors for bleeding in individual patients. These include optimizing blood pressure control to minimize the risk of ICH, avoiding unnecessary antiplatelet agents or nonsteroidal anti-inflammatory drugs, preventing gastrointestinal bleeding with proton pump inhibitors in patients at risk, implementing multidisciplinary interventions for falls prevention, and implementing dose reduction of DOACs when appropriate based on regular assessment of renal function and the potential for drug-drug interaction (12,23-25).
In conclusion, the study by van Rein et al. highlights a need to reduce the high bleeding rates associated with triple therapy in AF patients, especially in those with age ≥90 years, CHA2DS2-VASc score >6, or previous major bleeding. Progress has been made since the completion of their study, and guidelines have been altered based on recent trial findings and are now recommending DOAC-based dual antithrombotic therapy over VKA-based triple therapy for most AF patients undergoing PCI. It would be interesting to see what impact these recommendations will have on bleeding, ischemic events and mortality in clinical practice.
Acknowledgments
NC Chan is supported by a McMaster University Department of Medicine Internal Career Research Award.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace 2009;11:423-34. [Crossref] [PubMed]
- Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012;126:1185-93. [Crossref] [PubMed]
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Rothberg MB, Celestin C, Fiore LD, et al. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med 2005;143:241-50. [Crossref] [PubMed]
- Alexander JH, Lopes RD, Thomas L, et al. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2014;35:224-32. [Crossref] [PubMed]
- Shah R, Hellkamp A, Lokhnygina Y, et al. Use of concomitant aspirin in patients with atrial fibrillation: Findings from the ROCKET AF trial. Am Heart J 2016;179:77-86. [Crossref] [PubMed]
- Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013;127:634-40. [Crossref] [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- van Rein N, Heide-Jørgensen U, Lijfering WM, et al. Major Bleeding Rates in Atrial Fibrillation Patients on Single, Dual, or Triple Antithrombotic Therapy. Circulation 2019;139:775-86. [Crossref] [PubMed]
- Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500-10. [Crossref] [PubMed]
- Connolly SJ. Anticoagulant-Related Bleeding and Mortality. J Am Coll Cardiol 2016;68:2522-4. [Crossref] [PubMed]
- Held C, Hylek EM, Alexander JH, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J 2015;36:1264-72. [Crossref] [PubMed]
- Eikelboom JW, Connolly SJ, Hart RG, et al. Balancing the benefits and risks of 2 doses of dabigatran compared with warfarin in atrial fibrillation. J Am Coll Cardiol 2013;62:900-8. [Crossref] [PubMed]
- Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández AI, et al. Causes of Death in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol 2016;68:2508-21. [Crossref] [PubMed]
- Chan NC, Paikin JS, Hirsh J, et al. New oral anticoagulants for stroke prevention in atrial fibrillation: impact of study design, double counting and unexpected findings on interpretation of study results and conclusions. Thromb Haemost 2014;111:798-807. [Crossref] [PubMed]
- Lopes RD, Hong H, Harskamp RE, et al. Safety and Efficacy of Antithrombotic Strategies in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis of Randomized Controlled Trials. JAMA Cardiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [Crossref] [PubMed]
- Andrade JG, Verma A, Mitchell LB, et al. 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 2018;34:1371-92. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Mehta SR. Refining Antithrombotic Therapy for Atrial Fibrillation and Acute Coronary Syndromes or PCI. N Engl J Med 2019;380:1580-1. [Crossref] [PubMed]
- Chan NC, Eikelboom JW. How I manage anticoagulant therapy in older individuals with atrial fibrillation or venous thromboembolism. Blood 2019;133:2269-78. [Crossref] [PubMed]
- Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005;118:612-7. [Crossref] [PubMed]
- Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to Prevent Gastroduodenal Events in Patients Receiving Rivaroxaban and/or Aspirin in a Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology 2019;157:403-12.e5. [Crossref] [PubMed]


