
Managing intrinsic pro-degradatory mechanical and cellular cycles in the development of osteochondral lesions
Introduction
It is an aim of medical scientists to reverse disease. Chondral lesions that classically develop and super-specialised tissues have been the focus of many efforts in regenerative medicine to restore a biomechanically efficient, responsive, and self-sustaining equilibrium at the joint surface. One might target the articular cartilage. Alternatively, one may attempt to address the intrinsic capacity of a joint’s biochemical environment with molecules such as steroids, hyaluronic acid, or even a cocktail in platelet-rich plasma (PRP). Ultimately, success requires recognition that the treatment should target the joint as an integrated organ, rather than targeting the joint’s component tissues individually. Thus, therapy should have three components: the biology of the living, self-sustaining construct; the mechanics of this construct; and the physiological interaction between the two. Stem cells appear to have come some way toward addressing this therapeutic need, although we have yet to fully understand their capabilities and limitations. Capable of signalling and modulating their own behaviours and the behaviours of the tissues around them and capable of differentiating according to environmental, mechanical, and molecular triggers to produce a mechanically appropriate matrix, stem cells appear to be a panacea, with applications to the treatment of diverse pathologies. Yet while we seem to have a reservoir of this very special cell type within our tissues, not all stem cells are the same. In vitro studies suggest that umbilical cord-derived stem cells are especially potent, highlighting the importance of studying the therapeutic potential of this allogeneic tissue. As such, one might imagine these cells serve as factories delivering a persistent source of growth factors and other tissue-modulating molecules, but perhaps one should not rely on these cells to do any more than kick-start the intrinsic regenerative process. While one might hope that delivered autologous stem cells may eventually differentiate and potentially integrate into the host tissue (whether this actually happens is unclear), no such expectation is associated with use of allogeneic cells in most situations. The relative immuno-isolation afforded to the chondral tissue suggests that this tissue is an exception. Here, we assess whether we improve the symptoms of the organs we treat and correlate that with the benefits to the organs targeted.
Pathophysiology of the chondral lesion and targets for therapy
In living tissues, biomechanical homeostasis requires a responsive balance of catabolic and anabolic processes to counter the physical stresses to which the tissue is exposed. Physiological mechanical stresses trigger adaptive cellular responses, allowing tissues to develop a matrix that accommodates these stresses and efficiently dissipates the energy of the stress applied (1-3).
Supra-physiological stress may cause either direct mechanical injury to the tissue or trigger a remodelling process that includes catabolic degradation of the matrix (4). This process may be mediated by molecular signals from tissue macrophages and chondrocytes, including interleukins such as interleukin 7 (IL-7), which result in production of proteases [e.g., matrix metalloproteinases (MMPs)] by chondrocytes (5-7). Vascular endothelial growth factor (VEGF) is also released and induces further matrix loss (8). A chondral lesion becomes progressive when a catabolic process is triggered and negatively impacts the tissue’s ability to resist stress to the extent that subsequent physiological forces cause damage. Thus, the inter-relationships between biomechanical triggers and the adaptive or destructive response reaches either a physiological equilibrium or the progressive decline seen in degenerative arthropathy (Figure 1).
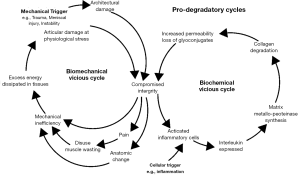
The intrinsic regenerative potential may be targeted by therapeutic measures. One may stimulate damaged osteochondral lesions through processes such as microfracture (9). One may attempt to directly treat the articular surface; autologous chondrocyte implantation (10) has evolved into measures that attempt chondroneogenesis by delivering matrix, cells, or both to create tissue de novo (11). Stem cells have multiple benefits in the regenerative process. Their potential to differentiate into a desired cell type has been described in experimental treatments. The previous use of such cells frequently required a period of subculture, single-stage applications using methods to extract relatively concentrated populations of adult autologous stem cells from bone marrow or adipose tissue have been described. These surgical approaches have evolved since Pridie’s drilling of osteochondral lesions; yet, they remain unable to reliably reverse the disease. At best, most delay an inevitable arthroplasty.
The use of intra-articular injections to ameliorate disease may provide a reasonably non-aggressive alternative to complex surgical procedures that have significant cost and morbidity. To prevent progression in a chondral lesion, it is necessary to target the loss of structural integrity and the cellular responses that combine to shift the tissue equilibrium toward degradation. One may target the mechanical triggers and structural deficits that weaken the force transfer capability of articular cartilage, causing further structural damage. Opportunities also exist in the modulation of pro-degradation interleukin-mediated catabolic processes triggered as part of the remodelling response.
Direct mechanical support
The most common injectable mechanical protection for the joint is hyaluronic acid. Its hydrophilicity and boundary lubricating properties (12) dissipate the energy of impact and shear on articular surfaces. Addition of phospholipids has also been studied, and the findings suggest that these might be useful in targeting the mechanical component of chondropathy (13). The intra-articular concentration will naturally decline, but the persistence of these molecules may be enhanced by increasing molecular weights. It may also be possible to sequester hyaluronic acid to the articular surface using customised peptide polymers (14).
Single-agent molecular immunomodulation
Classical modifiers include steroids and appear to have both positive and negative effects on remodelling processes. Hyaluronic acid is a ubiquitous structural molecule that can benefit multiple dimensions of the disease process. It appears to affect pain sensation (15) and has an immunological chondroprotective effect through its binding to cluster of differentiation 44 (CD44) to inhibit IL 1β and thus reduce MMP production and down regulate other inflammatory mediators, such as IL-8, IL-6, prostaglandin E2 (PGE2), and tumour necrosis factor α (TNFα) (16,17).
Multi-agent molecular immunomodulation
Platelets, exosomes, and conditioned media all deliver a number of physiologically active agents that can affect degenerative processes (18). These agents may have both pro-degradation (e.g., IL 1β, VEGF) (Pfander et al. 2001) and pro-synthetic actions [e.g., TGFβ, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF)], and fine tuning can be achieved to select one half of the balancing act that is cartilage matrix homeostasis.
Dynamic immunomodulation
The molecular immunomodulation methods described above lack persistence and responsiveness; the delivered agents disperse naturally and do not react to local signals. Cellular methods have the advantage of delivering a structure that can synthesise molecules as well as respond to mechanical and cytochemical triggers.
Stem cells offer an immuno-modulation capability that can also positively regulate intrinsic regenerative processes. Indeed, this can be achieved even if the delivered stem cells never reach the target tissue; for example, intravenously delivered stem cells have been shown to sequester the pulmonary vasculature (19), yet they are able to aid myocardial recovery (20). Similarly, one may expect intra-articular injection of such cells to deliver a localised, persistent source of intercellular signals and the ability to react to environmental signals; something that cannot occur by injecting molecular modulators of the intrinsic regenerative pathways.
The potency of umbilical cord-derived stem cells is said to far exceed that of adult stem cells. One can derive the cells directly from cord blood. A rich supply of such cells also exists in Wharton’s Jelly, which envelopes the vessels in the cord (21). Its application is almost universally allogeneic (22,23). Evidence suggests that umbilical cord-derived stem cells are hypo-immunogenic despite this, even though allogeneic adult stem cells may cause adverse reactions (24).
Conclusions
Articular cartilage homeostasis is responsible for maintaining mechanically efficient tissue and has multiple cellular contributors, including chondrocytes within the cartilage, synoviocytes, and cells in the subchondral bone. These link the mechanical stimulus and structural damage to processes that respond with both synthetic and lytic actions in a complex balancing act. In physiological conditions, this linkage restores the normal architecture and the energy dissipating capabilities of the joint surface. In pathological situations, the same linkage leads to the vicious cycles that characterise progressive degenerative arthropathy. This interrelationship implies that addressing one component in one episode may not be sufficient to restore damaged equilibrium. One needs to address both mechanical and cellular aspects of disease and to sustain this therapeutic measure until equilibrium is restored. The persistence of a therapeutic measure can be achieved by multiple applications of the chosen method or by delivering a living, active factory of disease-modifying agents, as in the use of stem cells. While evidence suggests that stem cells do work and do appear to provide sustained benefits, there is little evidence showing their persistence.
At a pragmatic level, there is a significant need for an off-the-shelf regenerative medicine solution. Autologous stem cells or PRP may be harvested trivially, but the high potency of umbilical cord-derived stem cells and their low immunogenicity and commercial availability indicate them as an interesting alternative. One needs to be sure, therefore, that bias has been eliminated before accepting the mainstream utility of this allogeneic tissue.
It is interesting that comparisons are often made between therapeutic measures that target different aspects of disease, when the measures investigated are not mutually exclusive. Intra-articular hyaluronic acid appears to target mechanical, enzymatic, and pain aspects of degenerative arthropathy, whereas stem cell therapies act through immunomodulation. It is the authors’ opinion that therapeutic measures should address both the hardware (the mechanics of joints) and the software (the cellular interactions in the joint) simultaneously to abolish the already activated degenerative cycles within a diseased joint. One might imagine that a combination of the two should also be included in such trials. Indeed, studies have combined the use of hyaluronic acid with stem cells and showed promising results (25).
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Jeffrey JE, Thomson LA, Aspden RM. Matrix loss and synthesis following a single impact load on articular cartilage in vitro. Biochim Biophys Acta 1997;1334:223-32. [Crossref] [PubMed]
- Steinmeyer J, Knue S. The proteoglycan metabolism of mature bovine articular cartilage explants superimposed to continuously applied cyclic mechanical loading. Biochem Biophys Res Commun 1997;240:216-21. [Crossref] [PubMed]
- Monfort J, Garcia-Giralt N, López-Armada MJ, et al. Decreased metalloproteinase production as a response to mechanical pressure in human cartilage: a mechanism for homeostatic regulation. Arthritis Res Ther 2006;8:R149. [Crossref] [PubMed]
- Blain EJ, Gilbert SJ, Wardale RJ, et al. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys 2001;396:49-55. [Crossref] [PubMed]
- Livne E, Laufer D, Blumenfeld I. Differential response of articular cartilage from young growing and mature old mice to IL-1 and TGF-beta. Arch Gerontol Geriatr 1997;24:211-21. [Crossref] [PubMed]
- Long D, Blake S, Song XY, et al. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthritis Res Ther 2008;10:R23. [Crossref] [PubMed]
- van Roon JA, Lafeber FP. Role of interleukin-7 in degenerative and inflammatory joint diseases. Arthritis Res Ther 2008;10:107. [Crossref] [PubMed]
- Pfander D, Körtje D, Zimmermann R, et al. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann Rheum Dis 2001;60:1070-3. [Crossref] [PubMed]
- Mithoefer K, Steadman JR. The microfracture technique. Tech Knee Surg 2006;5:140-8. [Crossref]
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889-95. [Crossref] [PubMed]
- Shetty AA, Kim SJ, Shetty V, et al. Autologous bone-marrow mesenchymal cell induced chondrogenesis: single-stage arthroscopic cartilage repair. J Tissue Eng Regen Med 2014;11:247-53. [Crossref]
- Mabuchi K, Obara T, Ikegami K, et al. Molecular weight independence of the effect of additive hyaluronic acid on the lubricating characteristics in synovial joints with experimental deterioration. Clin Biomech (Bristol, Avon) 1999;14:352-6. [Crossref] [PubMed]
- Kawano T, Miura H, Mawatari T, et al. Mechanical effects of the intraarticular administration of high molecular weight hyaluronic acid plus phospholipid on synovial joint lubrication and prevention of articular cartilage degeneration in experimental osteoarthritis. Arthritis Rheum 2003;48:1923-9. [Crossref] [PubMed]
- Faust HJ, Sommerfeld SD, Rathod S, et al. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 2018;183:93-101. [Crossref] [PubMed]
- Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res 1997;116:3-9. [Crossref] [PubMed]
- Julovi SM, Yasuda T, Shimizu M, et al. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum 2004;50:516-25. [Crossref] [PubMed]
- Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 2015;16:321. [Crossref] [PubMed]
- Zhang S, Chu W, Lai R, et al. Human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model. Cytotherapy. 2016;18:S13. [Crossref]
- Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009;18:683-92. [Crossref] [PubMed]
- Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009;5:54-63. [Crossref] [PubMed]
- Kim DW, Staples M, Shinozuka K, et al. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci 2013;14:11692-712. [Crossref] [PubMed]
- Sadlik B, Jaroslawski G, Puszkarz M, et al. Cartilage repair in the knee using umbilical cord Wharton’s jelly-derived mesenchymal stem cells embedded onto collagen scaffolding and implanted under dry arthroscopy. Arthrosc Tech 2017;7:e57-e63. [Crossref] [PubMed]
- Ha CW, Park YB, Chung JY, et al. Cartilage Repair Using Composites of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronic Acid Hydrogel in a Minipig Model. Stem Cells Transl Med 2015;4:1044-51. [Crossref] [PubMed]
- Joswig AJ, Mitchell A, Cummings KJ, et al. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther 2017;8:42. [Crossref] [PubMed]
- Park YB, Ha CW, Lee CH, et al. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med 2017;6:613-21. [Crossref] [PubMed]


