
Acute coronary syndrome and diabetic keto acidosis: the chicken or the egg?
Introduction
Myocardial infarction (MI) is a rare but well-known cause of diabetic decompensation. The frequency is of the order of 1%. Conversely, congestive heart failure and MI account for 28% of deaths in diabetic ketoacidosis (DKA) (1). The increase of troponin is a marker of myocardial necrosis, However, it is not limited to MI but also occurs in a variety of other conditions in ICU such as sepsis, pulmonary embolism, stroke, or renal failure without acute coronary syndrome (ACS) (1). DKA is a reported cause of troponin increase without ACS (2,3). In addition, the elevated troponin level is a predictor of poor outcome in patients admitted to intensive care for clinical conditions other than ACS (4). The two clinical cases presented below, demonstrate the various aspects of troponin increase in patients in DKA and are an opportunity to review the different mechanisms by which this occurs (Table 1).
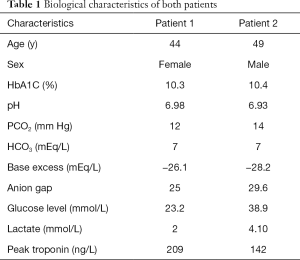
Full table
Case presentation
Case 1
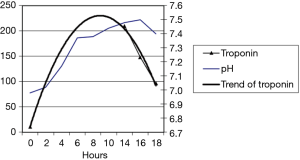
A 44-year-old female patient with type 1 diabetes was admitted to the ICU ward for DKA due to an inappropriate reduction of her insulin therapy. At 14 h post admission, a troponin elevation of 209 ng/L was observed without any chest pain (Figure 1). The electrocardiogram didn’t show a rise of the ST segment. Cardiac ultrasound did not show segmental dyskinesia. A coronary angiography performed after resolution of the acidosis revealed a sub-occlusion of the right coronary artery. The patient was therefore treated with angioplasty with stenting.
Case 2
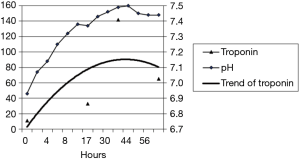
A 49-year-old male patient, suffering from type 1 diabetes, was admitted to ICU for DKA following an inappropriate insulin therapy justified by vomiting and abdominal pain. At 14 h post admission, the troponin level was at 33 ng/L and reached a maximum pic of 142 ng/L at 38 h post admission (Figure 2) without a history of chest pain. The electrocardiogram shows an elevation of the ST segment less than 2 mm from leads V1 to V3. An anterior apical dyskinesia was revealed by an ultrasound and the coronary angiography did not show any subsequently coronary anomaly.
Discussion
The interpretation of cardiac biomarkers is complex in DKA, which is due to the fact that the ACS can be the cause and also the consequence of the DKA. MI is the most common cause of death in DKA (5). Different hypotheses have been proposed to explain this phenomenon (Figure 3).
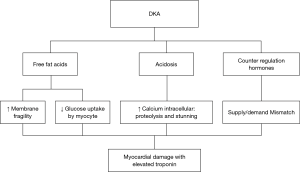
The interaction between acidosis and intracellular calcium
In a case report by Moller et al. two patients with elevated troponin levels had no abnormalities at the angiography and had a pH of 6.9 (3). Eubanks et al. have shown that a pH below 7.10 is an independent pronostic factor for troponin (cTnI) elevation on admission in DKA (6).
PH and intracellular calcium interact in complex ways. Following severe acidemia, intracellular calcium is increased by changes in transmembrane ionic motion at the sarcoplasmic membrane and sarcoplasmic reticulum (6). The rise of active intracellular calcium stimulates different biochemical pathways leading to proteolysis. Severe acidosis inhibits the interaction between calcium and contractile proteins, leading to myocardial stunning. Proteolysis and stunning both increase the serum level of cTnI (7).
The counter-regulatory hormones
Acute decompensation of diabetes is associated by a rise in counter-regulating hormones such as adrenaline, cortisol and glucagon (8,9). These hormones increase the oxygen demand of the myocardium (10). In diabetic patients with a history of coronary heart disease, coronary blood flow is compromised with an increase in supply-demand mismatch, resulting in myonecrosis with elevated cTnI (6).
The free fatty acid release
The release of free fatty acids was also observed in the acute diabetic decompensation (8,9). Free fatty acids are the precursors for hepatic ketone body formation. A high circulating level of free fatty acid leads to the incorporation of fatty acids into the lipid structure of the myocyte membrane with the formation of micelle with destabilization and rupture of this membrane (3,11).
Circulating insulin governs the extraction rate of glucose by cardiomyocytes (11). In case of DKA, insulin deficiency is associated with a high level of free fatty acids and ketone bodies, which inhibits the glucose uptake by the cell and thus deprives the myocardium of its energy substrate (12). In addition, the above-mentioned excess of catecholamines decreases the insulin reserves and increases the free fatty acid uptake by the myocardium, which is toxic to the myocardium (12). This phenomenon of cellular toxicity induces the cTnI production (10).
Conclusions
The troponin increase is a phenomenon described in patients with DKA and corresponds either to a pre-existing coronary pathology unmasked by a metabolic stress or to the toxicity of acidosis, the insulin deficiency or the presence of free fatty acids on the myocyte. A troponin elevation in a diabetic patient should always be considered a coronary abnormality until proven otherwise. The troponin kinetics, even delayed, does not allow us to distinguish if the cause is coronary or not.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
References
- Al-Mallah M, Zuberi O, Arida M, et al. Positive troponin in diabetic ketoacidosis without evident acute coronary syndrome predicts adverse cardiac events. Clin Cardiol 2008;31:67-71. [Crossref] [PubMed]
- Manikkan AT. Elevated Troponin I Levels in Diabetic Ketoacidosis Without Obstructive Coronary Artery Disease. J Endocr Soc 2018;2:1020-3. [Crossref] [PubMed]
- Moller N, Foss AC, Gravholt CH, et al. Myocardial injury with biomarker elevation in diabetic ketoacidosis. J Diabetes Complications 2005;19:361-3. [Crossref] [PubMed]
- Ammann P, Maggiorini M, Bertel O, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003;41:2004-9. [Crossref] [PubMed]
- Johnson DD, Palumbo PJ, Chu CP. Diabetic ketoacidosis in a community-based population. Mayo Clin Proc 1980;55:83-8. [PubMed]
- Eubanks A, Raza F, Alkhouli M, et al. Clinical significance of troponin elevations in acute decompensated diabetes without clinical acute coronary syndrome. Cardiovasc Diabetol 2012;11:154. [Crossref] [PubMed]
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol 1990;258:C967-81. [Crossref] [PubMed]
- Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335-43. [Crossref] [PubMed]
- Kitabchi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:2739-48. [Crossref] [PubMed]
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005;142:786-91. [Crossref] [PubMed]
- van der Vusse GJ, Glatz JF, Stam HC, et al. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 1992;72:881-940. [Crossref] [PubMed]
- Gandhi MJ, Suvarna TT. Cardiovascular Complications in Diabetic Ketoacidosis. Int J Diab Dev Countries 1995;15:132-3.


