
Increased risk of intracranial hemorrhage in preterm infants with OPRM1 gene A118G polymorphism
Introduction
Intracranial hemorrhage (ICH) is a common form of serious neonatal brain injury, which is more common in premature children. The smaller the gestational age (GA), the higher the morbidity and mortality, and severe cases can leave neurological sequelae. ICH is an important disability factor in neonates. With the development of perinatal medicine and neonatal intensive care technology, the survival rate of preterm infants has significantly increased. The ICH incidence rate remains high however, and a previous study reported that the incidence rate of preterm infants with periventricular-interventricular hemorrhage (IVH) is 9.3% in Chinese individuals, and even low-grade IVH has a significant impact on the neurodevelopmental outcome of preterm patients (1,2).
The A118G single nucleotide polymorphism (SNP) in the µ-opioid receptor gene OPRM1 has been well studied and shows a higher incidence in Asian populations. Additionally, this gene locus can change the amino acid sequence. Studies have confirmed (3) that A118G of µ-opioid receptor (OPRM1 A118G) is a meaningful mutation that changes µ-opioid receptor expression and peptide affinity and impacts opioid effects and µ-opioid-receptor-associated physiological functions. It has recently been confirmed that OPRM1 A118G is associated with a variety of disease susceptibilities, whereas the correlation between the OPRM1 AII8G polymorphism and neonatal diseases has not been reported.
The close association between the OPRM1 A118G SNP and neuropsychiatric factors has been widely recognized. The present study aims to discuss the relevance of OPRM1 A118G SNPs and ICH in preterm infants to explore the molecular genetic mechanisms of ICH and provide a reference for the clinically effective prevention and treatment of ICH.
Methods
Medical ethics
The present study obtained signed informed consent from the families of children, and the research plan was approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
The research objects
Inclusion criteria
(I) Premature infants of Han descent hospitalized during the period from July 2011 to March 2013 at the First Affiliated Hospital Of Zhengzhou University Neonatal Intensive Care Unit (NICU) were selected; (II) the subjects in the present study were consistent with the diagnostic criteria for preterm children, gestational week (GW) <37 weeks; (III) the ICH group of premature infants were diagnosed by cranial ultrasound (4); (IV) cases with complete data were also included.
Exclusion criteria
(I) Patients hospitalized after seven days of birth; (II) children from a mother with placenta previa or placental abruption; (III) patients complicated with severe infection or multiple organ failure after delivery; (IV) children with congenital malformations, hereditary metabolic diseases, and other diseases that may affect the blood system; (V) children with a history of anticoagulant drug use leading to a bleeding tendency after birth; (VI) pregnant mothers that did not receive perinatal care, or who had thrombocytopenia, bleeding disorders due to various reasons disease, or drug use that could cause drug bleeding tendencies.
Grouping
All subjects were analyzed by using bed cranial color Doppler ultrasound (Mindray, M5) within 3 days after birth, and cranial ultrasound examination by specially trained professionals. All test subjects were administered conventional supportive treatment measures. According to clinical manifestations and ultrasound examination results, preterm infants with ICH were considered as the hemorrhage group. During the same period, preterm infants with non-ICH due to premature birth and requiring hospitalization for observation were considered as the non-bleeding group. A total of 167 cases were included in the hemorrhage group, of which 99 were males and 68 females, the GA was 26–36+6 weeks, and the birth weight (BW) was 760–3,300 g. A total of 163 cases were included in the non-bleeding group, of which 91 were males and 75 females, the GA was 28+6–36 weeks, and the BW was 1,000–4,200 g.
Extraction of genomic DNA
A 2 mL femoral vein blood sample was obtained from every enrolled patient and subsequently stored in 2% EDTA anticoagulant tubes at 4 °C. Genomic DNA was isolated from leukocytes using a DNA extraction kit (Kangwei Century Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s instructions.
Polymerase chain reaction (PCR) amplification and enzyme reaction
The primers were designed according to the literature and gene library (5) by using the upstream 5'-GGTCAACTTGTCCCACTTAG and the downstream 5'-AATCACATACATGACCAGGAAGTTT-3' primers (Shanghai Sangon Biological Engineering Co., Ltd., Shanghai, China). A PCR reaction system contained 1 µL of 10 nmol/L of upstream and downstream primers, 2 µL of DNA template, 10 µL of 2× PCR Taq Mix, and 6 µL of ultrapure water to a final volume of 20 µL. The PCR reaction conditions included predenaturation at 94 °C for 3 min, followed by 38 cycles of denaturation at 94 °C for 30 s, annealing at 62 °C for 60 s, and extension at 72 °C for 60 s, with a final extension cycle at 72 °C for 10 min and a 4 °C hold. A 5-µL aliquot of the PCR product was subjected to 2% agarose gel electrophoresis to detect the PCR amplification products. The PCR reaction products were then subjected to restriction endonuclease digestion. The enzyme reaction system included the endonuclease 2 µL of Bsh1236I (Thermo Fisher Scientific, MA, USA), 2 µL of Buffer R (Thermo Fisher Scientific, Waltham, MA, USA), 10 µL of PCR product, and 18 µL of sterile deionized water in a 37 °C water bath for 12 h. After the digestion reaction was completed, polyacrylamide gel electrophoresis (PAGE) and silver staining were used to determine the obtained genotypes. A 5-µL aliquot of the digestion products was run on PAGE electrophoresis for 60 min (1× TBE electrophoresis buffer, voltage 5 V/cm), with molecular 100-bp DNA Ladder (Thermo Fisher Scientific, Waltham, MA, USA) as the standard, and subsequently, the gel was silver nitrate stained and analyzed to obtain the genotype results. The PCR reaction products were sequenced to verify the genotyping results.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software, and the genotype and allele frequencies in the two groups were calculated. The GA and BW of the two groups were analyzed using an independent sample t-test. The genotype and allele associated with the disease were tested using the χ2 test, with a test level α=0.05.
Results
General
The ICH group included 167 cases, of which 99 were males and 68 females, the average GA was 33.59±1.95 weeks, and the average BW was 1,849±578 g. The non-ICH group included 163 cases, of which 91 were male and 72 female preterm infants, with an average GA of 33.98±1.63 weeks and an average BW of 1,939±472 g.
The gender, GA, and BW comparisons between the two groups showed P values greater than 0.05, indicating that there was no statistically significant difference, and the two groups were comparable (Table 1).

Full table
PCR product detection
The PCR conditions described above were used to amplify a premature peripheral blood genomic OPRM1 gene of 193 bp in length. Agarose gel electrophoresis showed a specific 193 bp fragment, consistent with the expected amplified fragment length, which was used for the subsequent digestion reaction (Figure 1).
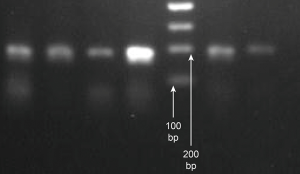
Endonuclease BstUI restriction results
The PCR reaction products of 193 bp in length included restriction enzymes BstUI of specific sites that can be digested to 169 and 24 bp fragments. The A/A type genotype is not cut, and gel electrophoresis showed a single 193 bp band; the G/G genotype can be cut to 169 and 24 bp fragments (the 24 bp band is too small to detect and is not clearly shown, thus only the 169 bp band was observed on the gel electrophoresis); the A/G genotype contained both 193 and 169 bp bands. Thus, PAGE- silver staining showed the three genotypes of OPRM1 A118G (Figure 2).
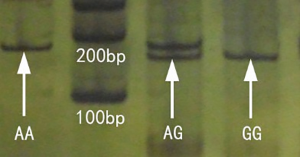
Gene sequencing
The PCR reaction products of different genotype groups were sequenced to verify the digestion typing results, as shown in Figure 3A (A/A), Figure 3B (A/G) and Figure 3C (G/G).
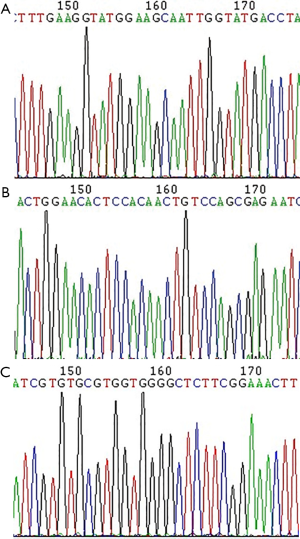
Analyzing the OPRM1 gene A118G loci polymorphism in the hemorrhage and non-bleeding groups
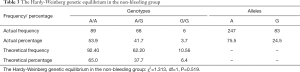
Examination of the Hardy-Weinberg equilibrium
The frequency distributions of the genotypes and alleles for A118G in the two groups are shown in Tables 2,3. The results indicate that the distribution of the genotypes and alleles in the two groups were consistent with the Hardy-Weinberg genetic equilibrium (P>0.05), and all subjects in the present study were randomly selected from large groups to represent the objective conditions of their respective groups.

Full table
Full table
Comparison of the OPRM1 gene A118G genotype and the allele frequency distribution in the hemorrhage and non-bleeding groups, as shown in Table 4
Full table
Comparison of the OPRM1 A118G allele frequency in the two groups was as follows: in the ICH group, the G/G genotype frequency (7.2%) was higher than that in the control group (3.7%), but there was no statistically significant difference between the two groups (chi-square =4.839, P=0.089). When the hybrid mutation type A/G was combined with homozygous mutation type G/G, the hemorrhage group (A/G + G/G) positive rate was higher than that in the control group, and this difference was statistically significant (chi-square =3.913, P=0.048). The allele frequencies of A and G were 68.3% and 31.7% respectively, in the hemorrhage group, and 75.5% and 24.5% respectively, in the control group. In the case group, the G allele frequency was increased, and this difference was statistically significant (chi-square =4.222, P=0.04) (Table 4). The risk of ICH in individuals carrying the G allele was 1.5 times greater than that for those carrying the A allele [odds ratio (OR): 1.549; 95% confidence interval (CI): 1.003–2.391].
Discussion
Neonatal ICH is a critical illness caused by a variety factors, and intracranial hypertension, convulsions, jaundice and respiratory failure are the main manifestations. ICH is both a common serious brain injury in newborns, especially in premature children; the younger the GA, the lower the BW, and the higher the incidence of ICH. The occurrence of neonatal ICH is closely related to neonatal anatomical physiology characteristics and a variety of perinatal high-risk factors. In addition, inflammatory reactions and endogenous factors, such as endogenous neurotransmitters, can also induce ICH. For instance, the release of endogenous opioid peptides can aggravate the damage (6,7).
In recent years, neural peptides have received increasing attention in studies of the mechanisms of cerebrovascular diseases. These studies have shown that opioid peptides and the opioid receptor system play important roles in the development of physiological and pathological processes in adult ischemic stroke (8,9). Additional studies have confirmed that opioid peptides, particularly the endogenous opioid peptide β-endorphin (β-EP), broadly participate in the pathophysiological process of neonatal diseases and are closely related to the development of neonatal diseases (10). When the body is in stress (such as in asphyxia, hypoxia, sepsis, or shock), pathological stressor stimuli, such as β-EP and ACTH, with origins in POMC, excited the hypothalamic-pituitary-adrenal axis, and ACTH secretion was increased, accompanied by the excessive release of β-EP. As an inhibitory regulator, β-EP reduced the sensitivity of carbon dioxide in brainstem neurons, induced respiratory depression, and increased the damage of cerebral edema and ICH, along with the secondary brain and other nervous system injuries that damage formation and development. The µ-opioid receptor is the primary site of the action of endogenous opioid peptides, and the loci of naloxone, nalmefene, and other opioid receptor antagonists in current clinical trials.
The µ-opioid receptor is a G protein-coupled receptor encoded by the µ-opioid receptor gene OPRM1. The OPRM1 has many gene SNPs. Hoehe et al. (11) identified at least 43 SNPs in the µ receptor gene, most of which are located in a non-coding region of this gene, and only a few SNPs in the coding region can cause changes in the amino acid sequence of the µ receptor. The A118G polymorphism, which can change the amino acid sequence of the gene and has a higher incidence in the population, is the most studied SNP. The All8G polymorphism involves the substitution of an N-terminal A exon by a G in the extracellular µ receptor, leading to 40-point mutations of aspartame phthalocyanine ammonia (Asn) to aspartic acid (Asp). Asp residue is the site of glycosylation in the µ receptor N-terminal region, and the loss of this site will seriously affect the receptor affinity and the ligand and receptor function efficiency. We also detected an important signaling mechanism in which β-EP binding to the All8G mutant induced a three-fold increase in the excitation current amplitude of the inward rectifier potassium channel. These preliminary findings support the view that different individuals react to endogenous opioid peptides, may be due to opioid receptor polymorphisms. Lötsch et al. (12) and other studies showed that individuals carrying a mutant allele G exhibited 1.74 times lower mitosis than wild-type homozygous individuals. Clinical studies have demonstrated that the application of opioid analgesic drugs to patients carrying the allele 118G significantly reduced the analgesic effect (13). In a study of the relationship between malignant tumor patients with the OPRM1 gene polymorphism and opioid analgesic requirements, Klepstad et al. (14) achieved the same analgesic effect in patients carrying the homozygous G allele by using more analgesic doses compared with wild-type homozygous patients. Using in vitro experiments, Bond et al. (15) confirmed that the affinity of opioid receptors and β-EP, and effectiveness in individuals carrying 118G allele homozygotes, is 3 times that of heterozygous receptors. This mechanism may function because the binding force of the receptor and the ligand depends on the molecular weight of the opioid peptide ligands. In addition to this, small molecule opioid peptides have no effect on the Al18G gene encoding receptor affinity, whereas macromolecular opioid peptides, such as β-EP, increase the affinity of these receptors.
Recent studies on OPRM1 A118G SNPs have primarily focused on the dependence of OPRM1 A118G and heroin, alcohol, and narcotic analgesics in pain tolerance, but the conclusions are not consistent. Research reports have shown that in Chinese individuals, the OPRM1 A118G genotype and allele frequencies were significantly different between substance-dependent patients and the normal population, and in heroin addicts, the G allele frequency was significantly higher than that in the control group, consistent with the findings of Bergen. Bond et al. (15) reported that the A118G (G/G) allele and β-EP affinity is three times that of the (A/A) allele, and carrying G allele increased an individual’s susceptibility to opioids and was a risk factor for substance dependence. However, some studies (16) have reported the opposite conclusion with the A allele being a possible risk factor for substance dependence, while other studies have shown no correlation between the 118 A/G mutation and opioid dependence (17).
The OPRM1 A118G mutation is the most common variant in the OPRM1 coding region, and studies have shown that the mutation frequency of OPRM1 A118G gene polymorphism is different in different populations. In Asian populations, the A118G mutation frequency was 35–47%, with the A118G mutation frequency being approximately 48.5% in Japanese individuals, 47% in Indian individuals, 45% in Malaysian individuals, 44% in Thai individuals, and 32.1% in Chinese individuals (18). In the present study, subjects were selected from Han Chinese preterm children, thus eliminating the impact of racial differences, and the study showed that the 118G allele frequency was 31.7%, consistent with the results described above. In the present study, the OPRM1 A118G gene locus gene frequencies in ICH and non-group ICH premature children were consistent with the Hardy-Weinberg equilibrium genetic laws, indicating that all of the subjects in the present study were randomly selected from large groups and can represent the objective situation of their respective groups.
Relevant studies have reported in their findings that the China normal crowd OPRM1 gene A118G A/A genotype frequency was 37.2–52.6%, A/G was 36.1–51.9%, and G/G was 10.9–11.3% (18,19). In the present study, the ICH and non-ICH group OPRM1 A118G A/A genotype frequencies were 43.7% and 54.6% respectively, whereas the frequencies of A/G were 49.1% and 41.7%, and G/G were 7.2% and 3.7%, respectively, consistent with the frequencies reported in the Chinese population. ICH OPRM1 gene mutation A118G polymorphism genotypes (A/G + G/G) positive rate was significantly higher than that of the non-ICH type (56.3%>45.4%), and the difference was statistically significant (χ2=3.913, P=0.048), indicating that the OPRM1 gene A118G polymorphism is associated with the occurrence of ICH. Due to differences in the ethnic and regional distribution of the China normal population OPRM1 118 A allele, the frequency was 64.9–70.6%, and the G allele frequency was 29.4–35.1% (18,19). The present study found that A allele frequencies of the ICH and non-ICH groups were 68.3% and 75.5% respectively, and OPRM1 118 G allele frequencies were 31.7% and 24.5% respectively. The study population G allele mutant frequency was basically consistent with that of the Chinese population, the G allele frequency of ICH was higher than that of the non-bleeding group, and the difference was statistically significant (χ2=4.222, P=0.040), indicating that the G allele may be a susceptibility gene in the occurrence of ICH. There are reports that the Indian allele G may have a protective effect on the heroin addict population (15). However, the present study showed that individuals carrying the allele G had a higher risk of ICH, and this risk was approximately 1.5-fold that of individuals carrying the allele A (OR: 1.549; 95% CI: 1.003–2.391); additionally, the OPRM1 gene 118G allele was positively correlated with the incidence of ICH, suggesting that the gene mutation may increase the risk of ICH. Further studies with expanded random testing sample sizes are needed to confirm the results.
The OPRM1 gene A118G polymorphism is a meaningful mutation related to the occurrence and susceptibility of many clinical diseases, but OPRM1 A118G polymorphism and neonatal disease correlations have not been reported. The present study showed that the OPRM1 A118G gene polymorphism is associated with ICH occurrence in premature infants, and carrying the 118G allele may increase the risk of ICH. OPRM1 A118G may be a potential susceptibility loci in ICH, and this knowledge may engender further insights into the genetic mechanisms for ICH in preterm infants. It may also provide a theoretical basis for the prevention and treatment of ICH in neonates, and set a new direction for the research and application of specific opioid receptor antagonists in newborns. However, more reliable large-sample, case-control studies are needed to support the results. Additionally, it is difficult to determine pathogenesis disease mechanisms in studies of single genes; therefore, additional in-depth and larger sample-sized research is needed to rule out accidental discovery. Genetic heritage research will be a substantial undertaking for the future, and studies from different levels and different perspectives, combined with joint multi-gene analysis, are still needed to obtain more valuable and comprehensive conclusions.
Conclusions
The OPRM1 gene A118G polymorphism is associated with ICH in premature infants. The OPRM1 Gene A118G may be a sensitive gene leading to the occurrence of ICH.
Acknowledgments
Funding: The study was supported by the Henan Science and Technology Research Project (International Science and Technology Cooperation Field), Number: 172102410017.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study obtained signed informed consent from the families of children, and the research plan was approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
References
- Subspecialty Group of Neonatology, Society of Pediatrics, Chinese Medical Association, et al. Multicenter survey for incidence of intraventricular hemorrhage in premature infants in seven big cities of China. Zhonghua Er Ke Za Zhi 2009;47:5-11. [PubMed]
- Klebermass-Schrehof K, Czaba C, Olischar M, et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs Nerv Syst 2012;28:2085-92. [Crossref] [PubMed]
- Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology 2008;109:520-6. [Crossref] [PubMed]
- Volpe JJ. Intraventricular hemorrhage and brain injury in the premature infant. Diagnosis, prognosis, and prevention. Clin Perinatol 1989;16:387-411. [Crossref] [PubMed]
- Romberg RR, Olofsen E, Bijl H, et al. Polymorphism of mu-opioid receptor gene (OPRM1:c.118A>G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology 2005;102:522-30. [Crossref] [PubMed]
- Hayes RL, Galinat BJ, Kulkarne P, et al. Effects of naloxone on systemic and cerebral responses to experimental concussive brain injury in cats. J Neurosurg 1983;58:720-8. [Crossref] [PubMed]
- Zhou Y, Fathali N, Lekic T, et al. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke 2011;42:439-44. [Crossref] [PubMed]
- Laungani SG, Delivoria B, Gintzler A, et al. Apgar scores and cerebrospinal fluid beta-endorphinlike immunoreactivity during the first day of life. Preliminary observations. Am J Dis Child 1985;139:403-4. [Crossref] [PubMed]
- Storm H, Stoltenberg L, Saugstad OD, et al. Beta-endorphin immunoreactivity levels in CSF after laryngeal chemoreflex activation correlate with apnoea duration in piglets. J Perinat Med 1996;24:363-72. [Crossref] [PubMed]
- Weller A, Feldman R. Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides 2003;24:779-88. [Crossref] [PubMed]
- Hoehe MR, Köpke K, Wendel B, et al. Sequence variability and candidate gene analysis in complex disease: association of mu opioid receptor gene variation with substance dependence. Hum Mol Genet 2000;9:2895-908. [Crossref] [PubMed]
- Lötsch J, Skarke C, Grösch S, et al. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics 2002;12:3-9. [Crossref] [PubMed]
- Lötsch J, Skarke C, Liefhold J, et al. Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet 2004;43:983-1013. [Crossref] [PubMed]
- Klepstad P, Rakvåg TT, Kaasa S, et al. The 118 A > G polymorphism in the human mu-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand 2004;48:1232-9. [Crossref] [PubMed]
- Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A 1998;95:9608-13. [Crossref] [PubMed]
- Strong JA, Dalvi A, Revilla FJ, et al. Genotype and smoking history affect risk of levodopa-induced dyskinesias in Parkinson's disease. Mov Disord 2006;21:654-9. [Crossref] [PubMed]
- Crowley JJ, Oslin DW, Patkar AA, et al. A genetic association study of the mu opioid receptor and severe opioid dependence. Psychiatr Genet 2003;13:169-73. [Crossref] [PubMed]
- Tan EC, Tan CH, Karupathivan U, et al. Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport 2003;14:569-72. [Crossref] [PubMed]
- Szeto CY, Tang NL, Lee DT, et al. Association between mu opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport 2001;12:1103-6. [Crossref] [PubMed]


