
Expanding congenital abnormalities of the kidney and urinary tract (CAKUT) genetics: basonuclin 2 (BNC2) and lower urinary tract obstruction
Chronic kidney disease (CKD): the 21-century epidemic
CKD is characterized by chronic (>3 months), often irreversible, evidence of kidney injury or dysfunction with consequences for health (1). A diagnosis is made in the presence of decreased glomerular filtration rate (GFR) or if there is analytical (most commonly pathological albuminuria), histological or imaging evidence of kidney injury. The criterion “with consequences for health” implies that CKD is associated with an increased risk of progression to end-stage renal disease (ESRD) requiring renal replacement therapy, which is the best-known consequence of CKD. However, CKD is also associated with an increased risk for premature death and in fact, CKD is projected to become 1 of the top 5 causes of death in the world by 2040 and similar trends have been described in individual countries (2,3). While the most frequent causes of CKD are acquired, including kidney disease secondary to diabetes and hypertension, the influence of genetic factors has been increasingly recognized, including genetic defects leading to congenital abnormalities of the kidney and urinary tract (CAKUT). CAKUT may be caused by hereditary genetic defects, as recently exemplified by the description of basonuclin 2 (BNC2) nonsense variants as causing congenital lower urinary-tract obstruction (LUTO) (4).
What is CAKUT?
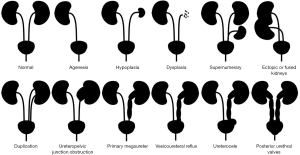
In PubMed, publications using the term CAKUT date from 1999, 20 years ago (5). CAKUT is considered the leading cause of pediatric ESRD and the most common cause of CKD before 30 years of age (6). The spectrum of anomalies includes kidney abnormalities (agenesis, hypoplasia or dysplasia as well as supernumerary, ectopic or fused kidneys) and urinary tract abnormalities (e.g., ureter duplication, ureteropelvic junction obstruction, primary megaureter or ureterovesical junction obstruction, vesicoureteral reflux, ureterocele, and posterior urethral valves, which are a cause of LUTO) (Figure 1). Genetic defects have been increasingly recognized as causing CAKUT. However, monogenic mutations currently explain only 14% of CAKUT cases (7). In addition to classical gene variants in developmental genes (missense or nonsense mutations, deletions, frameshift mutations), the spectrum of genetic defects causing CAKUT keeps expanding. Thus, CAKUT may also result from copy number variations (CNV) and mutations in genes, such as SON, regulating the splicing of CAKUT-causing genes (8,9). There is even a genotype-phenotype correlation at this level, with kidney anomaly cases being most enriched for exonic CNVs (8). Interestingly, genomic disorders causing CAKUT may also increase the risk of neurocognitive impairment, whose early recognition can impact clinical care (10). CAKUT may also form part of a spectrum of extrarenal and kidney abnormalities with very variable expression in terms of frequency, severity and type of CAKUT (11). Hypospadias and LUTO are also part of the CAKUT, spectrum. Hypospadias is a common congenital anomaly of the external male genitalia, in which the urethral meatus is abnormally placed in a ventral position. The pathogenesis is considered multifactorial: it may be influenced by environmental factors that negatively affect androgenic stimulation, but it may be related to single gene mutations (12). Hypospadias may be part of syndromes associated with other CAKUT, tumors and other systemic manifestations; and may present as non-syndromic hypospadias (12). Hypospadias is not usually associated with progressive CKD (13). However, LUTO may be associated with progressive CKD. LUTO is a rare condition characterized by obstruction of the bladder outflow, leading to secondary retrograde dilatation of the urinary tract. The diagnosis may be made in utero, especially if severe, in childhood or, for milder forms, in adulthood, when it presents as repeated urinary tract infections. The most common anatomical cause of LUTO is the presence of posterior urethral valves that occurs only in males, at the level of the prostatic urethra. Another less frequent cause of LUTO is urethral atresia, which can occur in both sexes (4).
What are zinc finger proteins?
Zinc finger proteins are transcription factors that regulate gene expression by binding to DNA and regulate numerous physiological processes like cell proliferation, differentiation and apoptosis (14). Zinc finger proteins are characterized by the presence of zinc fingers. A zinc finger is a small protein structural motif that contains a zinc ion and binds specific DNA sequences known as GC boxes (14,15). The zinc ion is ligated to a combination of cysteines and histidines, thus stabilizing the folds of the fingers (16). Different types of fingers are recognized based on the number and order of these amino acids. Cys2His2 is the classic zinc finger, characterized by two cysteines in one chain and two histidines in other (14). Zinc finger proteins may also be classified according to their overall shape into Cys2His2-like, treble clef, and zinc ribbon (17). The crystallographic structure of the classical zinc finger has two β-sheets and one α-helix (14). A few amino acids in the α-helix that juxtaposes three base pairs on DNA confer the DNA binding specificity (14).
What is BNC2?
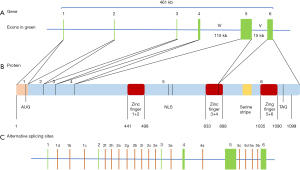
BNC2 is an extremely conserved Cys2His2 zinc finger protein orthologous to BNC1 (18). Genes encoding both proteins differ in size and are located on different chromosomes, but they have a common evolutionary origin and BNC2 is thought to be older and to have remained largely invariant retaining its original function (19,20). The BNC2 gene is located at 9p22 and contains six exons encoding a 1,099-residue protein with three pairs of zinc fingers, a putative nuclear location signal (NLS) and a serine stripe (19,21) (Figure 2). The 15th exon encodes the NLS and the first three zinc fingers and a part of the 4th zinc finger and the 6th exon encodes the remaining part of the 4th finger and the 5th and 6th zinc fingers (22). However, BNC2 may undergo alternative splicing with 23 alternative exons and has the potential to generate 89,468 mRNA isoforms (22). This huge number of potential isoforms and the presence of multiple zinc finger pairs, each potentially binding to a different target sequence, may explain the pleiotropic effects of BNC2 (18,21). BNC2 localizes in nuclear speckles and has an additional presumed function in nuclear pre-mRNA processing (18,19). The tissue distribution of BNC2 is wide and it is abundant in testis, skin, kidney, uterus and intestine (21). In addition, to the disease associations, discussed below, in male gonocytes, BNC2 represses meiosis and mitosis and also regulates hair follicle cycles (23).
What are the disease associations of BNC2?
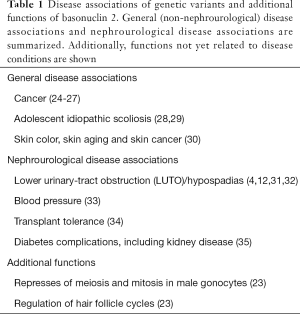
Genetic variants in BNC2 have been associated with human disease, and in some cases, the relationship has been very well documented in functional animal studies (Table 1). BNC2 single nucleotide polymorphisms (SNPs) have been associated with adolescent idiopathic scoliosis (28). At least one of the SNPs is functional and the susceptibility allele was associated with both higher binding to a transcription factor, YY1 (yin and yang 1), and higher BNC2 enhancer activity than the non-susceptibility allele. Furthermore, BNC2 overexpression produced body curvature in developing zebrafish, supporting the relevance of the findings (29). Finally, BNC2 loss-of-function mutations have been identified in diverse cancers (24). Although a tumor suppressor role has been proposed, the molecular mechanisms are unclear. Recently, BNC2 overexpression was shown to upregulate interferon-stimulated and tumor suppressor genes and to cause growth arrest of cancer cells (25,26) while BNC2 downregulation increased cancer cell survival (27).
Full table
Disruption of the Bnc2 gene in mice causes neonatal death with cleft palate and craniofacial abnormalities (23). Genetic BNC2 variants have been also associated with systolic blood pressure, the renal and retinal complications of diabetes (35), skin pigmentation (30) and better tolerance to liver and kidney transplantation (34).
What is the relationship between BNC2 and lower urinary tract obstruction and hypospadias?
However, until now, the most relevant disease association of BNC2 was a form of CAKUT, non-syndromic distal hypospadias (12,31,32). Non-synonymous variations in BNC2 gene were found in 12.5% of American patients with hypospadias (32). Heterozygous pathogenic mutations in BNC2 where found in Japanese and Vietnamese patients (31). In this regard, Bnc2−/− mice displayed a high frequency of distal urethral defects that were also observed but with reduced penetrance in Bnc2+/− mice (32). In this regard, BNC2 is involved in urethral development. Preclinical data in newborn mice have demonstrated a high BNC2 expression in periurethral tissue (32). In a 7-week embryo, immunohistochemistry localized high BNC2 expression to the urogenital sinus, the precursor of the bladder and urethra, and, using in situ hybridization, high BNC2 expression was demonstrated during lower urinary tract development (4). A high BNC2 expression is also observed in adult male urethra (4). Only recently, Kolvenbach et al. identified a truncating mutation in a family of four affected and a missense variant in a family of two affected members with LUTO with an autosomal dominant inheritance and varying degrees of phenotypic manifestations (4). Upon this finding, they re-sequenced 14 known BNC2 transcripts in 697 patients with LUTO in the AGORA study of patients and from a multinational collaboration, and found a probably pathogenic BNC2 variant and two variants of uncertain clinical significance in patients with urethral stenosis or posterior urethral valves (4). The hypothesis that BNC2 disruption indeed causes LUTO was tested in zebrafish, whose embryos expressed bnc2 in the pronephric duct and cloaca, analogs of the mammalian lower urinary tract. Indeed, zebrafish bnc2 knockdown using different methods caused pronephric-outlet obstruction and cloacal dilatation, thus phenocopying human congenital LUTO, and this was rescued by wild-type but not by mutated human BNC2 mRNAs (4). Thus, Kolvenbach et al. have identified and characterized clear pathogenic gene variants causing LUTO with urethral blockade, but were unable to progress in identifying the molecular pathways leading to LUTO or the factors influencing the incomplete penetrance.
What else needs to be known?
Genetic variants in BNC2 were identified as causing a specific form of CAKUT, LUTO. This will allow to screen for the risk of LUTO in predisposed families. However, the real challenge would be to develop new therapeutic approaches for LUTO or for other BNC2-associated diseases, based on this new knowledge. The answer to some question may allow to progress in this aim: what are the specific BNC2 isoforms implicated? What are the gene targets that are disrupted? And the cell processes involved? What background, genetic or environmental, influences the incomplete penetrance? How can BCN2 dysfunction be targeted during or after development?
Acknowledgments
Funding: This work was supported by FIS PI16/02057, PI19/00588, PI19/00815, DTS18/00032, REDinREN RD016/0009 Fondos FEDER, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071), Sociedad Española de Nefrología, FRIAT, and Comunidad de Madrid B2017/BMD-3686 CIFRA2 and Rio Hortega to MV Perez-Gomez.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E, et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019;12:258-61. [Crossref] [PubMed]
- Ortiz A, Sanchez-Niño MD, Crespo-Barrio M, et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: Keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019;39:29-34. [Crossref] [PubMed]
- Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018;392:2052-90. [Crossref] [PubMed]
- Kolvenbach CM, Dworschak GC, Frese S, et al. Rare Variants in BNC2 Are Implicated in Autosomal-Dominant Congenital Lower Urinary-Tract Obstruction. Am J Hum Genet 2019;104:994-1006. [Crossref] [PubMed]
- Nishimura H, Yerkes E, Hohenfellner K, et al. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell 1999;3:1-10. [Crossref] [PubMed]
- Jain S, Chen F. Developmental pathology of congenital kidney and urinary tract anomalies. Clin Kidney J 2018;12:382-99. [Crossref] [PubMed]
- van der Ven AT, Connaughton DM, Ityel H, et al. Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract. J Am Soc Nephrol 2018;29:2348-61. [Crossref] [PubMed]
- Verbitsky M, Westland R, Perez A, et al. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet 2019;51:117-27. [Crossref] [PubMed]
- Kim J-H, Park EY, Chitayat D, et al. SON haploinsufficiency causes impaired pre-mRNA splicing of CAKUT genes and heterogeneous renal phenotypes. Kidney Int 2019;95:1494-504. [Crossref] [PubMed]
- Sanna-Cherchi S, Westland R, Ghiggeri GM, et al. Genetic basis of human congenital anomalies of the kidney and urinary tract. J Clin Invest 2018;128:4-15. [Crossref] [PubMed]
- Madariaga L, García-Castaño A, Ariceta G, et al. Variable phenotype in HNF1B mutations: extrarenal manifestations distinguish affected individuals from the population with congenital anomalies of the kidney and urinary tract. Clin Kidney J 2018;12:373. [Crossref] [PubMed]
- van der Zanden LF, van Rooij IA, Feitz WF, et al. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update 2012;18:260-83. [Crossref] [PubMed]
- Baskin L. What Is Hypospadias? Clin Pediatr (Phila) 2017;56:409-18. [Crossref] [PubMed]
- Cassandri M, Smirnov A, Novelli F, et al. Zinc-finger proteins in health and disease. Cell Death Discov 2017;3:17071. [Crossref] [PubMed]
- Klug A, Rhodes D. Zinc fingers: a novel protein fold for nucleic acid recognition. Cold Spring Harb Symp Quant Biol 1987;52:473-82. [Crossref] [PubMed]
- Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem 2010;79:213-31. [Crossref] [PubMed]
- Krishna SS, Majumdar I, Grishin NV. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res 2003;31:532-50. [Crossref] [PubMed]
- Vanhoutteghem A, Messiaen S, Herve F, et al. The zinc-finger protein basonuclin 2 is required for proper mitotic arrest, prevention of premature meiotic initiation and meiotic progression in mouse male germ cells. Development 2014;141:4298-310. [Crossref] [PubMed]
- Vanhoutteghem A, Djian P. Basonuclins 1 and 2, whose genes share a common origin, are proteins with widely different properties and functions. Proc Natl Acad Sci USA 2006;103:12423-8. [Crossref] [PubMed]
- Vanhoutteghem A, Maciejewski-Duval A, Bouche C, et al. Basonuclin 2 has a function in the multiplication of embryonic craniofacial mesenchymal cells and is orthologous to disco proteins. Proc Natl Acad Sci USA 2009;106:14432-7. [Crossref] [PubMed]
- Vanhoutteghem A, Djian P. Basonuclin 2: An extremely conserved homolog of the zinc finger protein basonuclin. Proc Natl Acad Sci USA 2004;101:3468-73. [Crossref] [PubMed]
- Vanhoutteghem A, Djian P. The human basonuclin 2 gene has the potential to generate nearly 90,000 mRNA isoforms encoding over 2000 different proteins. Genomics 2007;89:44-58. [Crossref] [PubMed]
- Vanhoutteghem A, Delhomme B, Hervé F, et al. The importance of basonuclin 2 in adult mice and its relation to basonuclin 1. Mech Dev 2016;140:53-73. [Crossref] [PubMed]
- Goode EL, Chenevix-Trench G, Song H, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet 2010;42:874-9. [Crossref] [PubMed]
- Urgard E, Reigo A, Reinmaa E, et al. Human basonuclin 2 up-regulates a cascade set of interferon-stimulated genes with anti-cancerous properties in a lung cancer model. Cancer Cell Int 2017;17:18. [Crossref] [PubMed]
- Akagi T, Ito T, Kato M, et al. Chromosomal abnormalities and novel disease-related regions in progression from Barrett’s esophagus to esophageal adenocarcinoma. Int J Cancer 2009;125:2349-59. [Crossref] [PubMed]
- Cesaratto L, Grisard E, Coan M, et al. BNC2 is a putative tumor suppressor gene in high-grade serous ovarian carcinoma and impacts cell survival after oxidative stress. Cell Death Dis 2016;7:e2374. [Crossref] [PubMed]
- Ogura Y, Takeda K, Kou I, et al. An international meta-analysis confirms the association of BNC2 with adolescent idiopathic scoliosis. Sci Rep 2018;8:4730. [Crossref] [PubMed]
- Ogura Y, Kou I, Miura S, et al. A Functional SNP in BNC2 Is Associated with Adolescent Idiopathic Scoliosis. Am J Hum Genet 2015;97:337-42. [Crossref] [PubMed]
- Gao W, Tan J, Hüls A, et al. Genetic variants associated with skin aging in the Chinese Han population. J Dermatol Sci 2017;86:21-9. [Crossref] [PubMed]
- Kon M, Suzuki E, Dung VC, et al. Molecular basis of non-syndromic hypospadias: systematic mutation screening and genome-wide copy-number analysis of 62 patients. Hum Reprod 2015;30:499-506. [Crossref] [PubMed]
- Bhoj EJ, Ramos P, Baker LA, et al. Human balanced translocation and mouse gene inactivation implicate Basonuclin 2 in distal urethral development. Eur J Hum Genet 2011;19:540-6. [Crossref] [PubMed]
- Li C, He J, Chen J, et al. Genome-Wide Gene-Potassium Interaction Analyses on Blood Pressure: The GenSalt Study (Genetic Epidemiology Network of Salt Sensitivity). Circ Cardiovasc Genet 2017. [Crossref] [PubMed]
- Roedder S, Li L, Alonso MN, et al. A Three-Gene Assay for Monitoring Immune Quiescence in Kidney Transplantation. J Am Soc Nephrol 2015;26:2042-53. [Crossref] [PubMed]
- Paterson AD, Waggott D, Boright AP, et al. A Genome-Wide Association Study Identifies a Novel Major Locus for Glycemic Control in Type 1 Diabetes, as Measured by Both A1C and Glucose. Diabetes 2010;59:539-49. [Crossref] [PubMed]


