
Atorvastatin suppresses vascular hypersensitivity and remodeling induced by transient adventitial administration of lipopolysaccharide in rats
Introduction
Blood vessel walls are composed of the intima, media, and adventitia. The media is mainly composed of vascular smooth muscle cells (VSMCs) in an extracellular matrix (ECM). VSMCs are not terminally differentiated and possess the potential to change phenotype in response to alterations in the local environment (1). Phenotypic switching of VSMCs from a differentiated (contractile) to a dedifferentiated (proliferative) state plays a critical role in the pathophysiology of cardiovascular diseases, including atherosclerosis, hypertension, and restenosis after angioplasty (1,2). The transition of VSMCs between contractile and proliferative phenotypes is influenced by microenvironmental cues, such as growth factors, ECM components, cell-cell contacts, and neuronal inputs, which lead to alterations in the expression of stereotypical VSMC genes.
Myocardin is a transcriptional coactivator of serum response factor (SRF), which controls the expression of most genes for smooth muscle (SM) contractile proteins (3). As a master coactivator of SRF, myocardin plays a critical role in the transcription of SM contractile genes, such as SM α-actin, SM22, calponin, SM myosin heavy chain (SM-MHC), and SM myosin light chain kinase (SM-MLCK) (4-6), which presumably increases the contractility of cardiac and SM. Deregulation of myocardin protein and/or activity has been implicated in numerous cardiovascular diseases, including atherosclerosis and restenosis (7-9).
RhoA is a member of a small monomeric GTPases family that is involved in SM contraction and has been implicated in certain SM-dependent processes, such as cell proliferation, adhesion, motility, and migration (10-12). The best-characterized downstream effectors of RhoA are Rho-associated, coiled-coil-containing protein kinases (ROCKs), which are directly involved in SM contraction (13). RhoA/ROCK signaling induces myocardin and subsequently stimulates the transcription of SM-specific genes, such as SM-α actin, SM-MHC, and desmin (14,15).
For many years, atherosclerosis has been regarded as an inflammatory disease (16). As arterial injuries are generally initiated at the interface with circulating blood, most studies have focused on the innermost intimal layer rather than on the outermost adventitial layer. However, increasing evidence suggests that the adventitia is a mediator of atherosclerosis and vascular dysfunction (17-19). Additionally, adventitial inflammation occurs in blood vessels of atherosclerosis patients and the infiltration of inflammatory cells into the vascular adventitia is positively correlated with vascular membrane lesions of atherosclerosis (17-19).
Here, we directly added the bacterial product lipopolysaccharide (LPS) to the adventitia of rat carotid arteries to induce transient adventitial inflammation. LPS-induced transient adventitial inflammation resulted in vascular hypersensitivity to serotonin, proliferation, and disordered arrangement of VMSCs. We also demonstrated that LPS addition activated the RhoA/Rho-kinase ROCK2. Importantly, fasudil and atorvastatin inhibited ROCK2, completely blocked hypercontraction in response to serotonin, and prevented the proliferation and disordered rearrangement of VSMCs. Our findings indicate that the activation of the RhoA/Rho-kinase signaling pathway plays an important role in LPS-induced alteration of vascular contractility and VSMC proliferation. Both fasudil and atorvastatin prevented the LPS-induced increases in myocardin levels, suggesting the involvement of ROCK2 activation in induced myocardin expression. In summary, our data indicated that transient adventitial inflammation caused proliferation and disordered arrangement of VSMCs, as well as dysregulated expression of some stereotypical VSMC genes. Additionally, activation of the RhoA/Rho-kinase signaling pathway played an important role in the LPS-induced alteration of vascular contractility and proliferation.
Methods
The experimental protocols for animal use and care were approved by the Animal Care and Use Committee of China Medical University. All Male Wistar rats used in experiments were obtained from the Laboratory Animal Center of China Medical University. All animal experiments were conducted in strict accordance with the university guidelines. All surgeries were performed under anesthesia by intraperitoneal injection of chloral hydrate (30 mg/kg), and all efforts were made to minimize suffering throughout the course of the study.
Male 15-week-old Wistar rats (300–350 g) were randomly assigned to four groups with six rats in each group. In groups I and II, rats were administered distilled water (1 mL/kg, intragastrically). In group III, rats received fasudil (15 mg/kg, intraperitoneally). In group IV, rats were treated with atorvastatin (30 mg/kg, intragastrically). All treatments were given daily for 11 days. On day 8, both sides of the common carotid artery of each rat from each group were separated surgically and cleared of adherent connective tissue along the longitudinal axis (15–18 mm in length). The exposed bilateral common carotid artery adventitia was then infused with 5 µL sterile saline in group I rats, and with 5 µL sterilized LPS (10 µg/mL) in group II–IV rats. In brief, groups I–IV were termed as control (I), LPS (II), fasudil + LPS (III), and atorvastatin + LPS (IV) groups.
On day 8–13, the left common carotid and right femoral arteries of rats from each group were exposed surgically and a transonic volume flow-probe (2PSB; Transonic Systems Inc., Ithaca, NY, USA) and a hand-made tube were placed around the carotid or femoral artery, respectively. Carotid blood flow and femoral blood pressure were measured using a transonic perivascular flowmeter (TS420; Transonic Systems Inc., Ithaca, NY, USA) and recorded using a computerized acquisition system (MP150; Biopac Systems, Goleta, CA, USA). The system provided real-time volume flow measurements with a resolution of 0.05 mL/min. The carotid was minimally manipulated to avoid vasospasm. Vascular responses were examined after the topical application of serotonin. The carotid artery was gently wrapped with a cotton mesh and 5 µL 1% serotonin was administered flush to the cotton mesh. Carotid artery responses to serotonin were expressed as the percent changes of blood flow induced by serotonin compared with the resting blood flow. Then, rats were humanely killed with an overdose of chloral hydrate and the left carotids were excised and fixed in 4% paraformaldehyde for 24 h. Arterial segments were embedded in paraffin and cut into 4-µM-thick sections at the midpoint of the common carotid.
Morphological analyses were performed on hematoxylin and eosin stained (H&E stained) paraffin-embedded sections of the previously described arterial segments using a light microscope coupled to a digital camera (BX51; Olympus, Japan) imaging system. The thickness of intima and media were measured using DP2-BSW software (Olympus, Japan).
Total RNA was isolated from the pooled arteries (n=6 per group) using TRIzol reagent (Invitrogen Co., Carlsbad, CA, USA). Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed as previously reported (18). The following primers were used: myocardin, (F) 5'-GCCTACCGTATTCCTATTAAAGC-3' and (R) 5'-AGAGACTCGGGCAATCTGTGT-3'; myosin phosphatase target subunit 1 (MYPT1), (F) 5'-GCTTCCAGAACATACGATGAGAC-3' and (R) 5'-TACGGGAGTAGGCAGAGGTT-3'; ROCK2, (F) 5'-AACAGTCCGTGGGTGGTTCA-3' and (R) 5'-CGTTATCGGGCTTCACATCTC-3'; tumor necrosis factor α (TNFα), (F) 5'ACCACCAAGTGGAGGAGCAG-3' and (R) 5'-TGTCCCTTGAAGAGAACCTG-3'; interleukin-6 (IL-6), (F) 5'-GTGCAATGGCAATTCTGATTG-3' and (R) 5'-TAGCTATGGTACTCCAGAAG-3'; and β-actin, (F) 5'-CTCATCCACGA AACCACCTAT-3' and (R) 5'-CGCCGATCCAGACAGAATA-3'. Results were normalized to β-actin expression levels and presented as arbitrary units.
The pooled carotids arteries (n=6 per group) were lysed using lysis buffer [20 mM Tris-HCl, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid (EDTA), and 1% Triton X-100] containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Extracted proteins were quantified using the bicinchoninic acid (BCA) protein assay kit. Equivalent amounts of protein (50 µg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). Western blotting was performed using primary antibodies against ROCK2 (Abcam, Cambridge, UK), MYPT1 (pThr696) (Abcam, Cambridge, UK), myosin light chain (MLC) (pSer19, CST, Danvers, MA, USA), myocardin (Abbiotec, San Diego, CA, USA), SM-α actin (Abcam, Cambridge, UK), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Millipore, Billerica, MA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse were used as secondary antibodies (Amersham Biosciences, Amersham, UK). Immunoreactivity was visualized using an enhanced chemiluminescence (ECL) western blotting detection kit (Millipore, Billerica, MA, USA). Quantitative assessments of band densities were performed by scanning densitometry.
The ROCK2 kinase activity of the pooled carotids arteries (n=6 per group) were measured using tissue ROCK2 kinase activity quantitative assay kit (GenMed Scientifics Inc., USA) according to the manufacture’s instruction.
All results are expressed as the mean ± standard deviation (SD) or the mean ± standard error of the mean (SEM) where appropriate. Comparisons were made by one-way analysis of variance with Bonferroni’s post hoc test. A value of P<0.05 was considered to represent a statistically significant difference.
Results
Microscopic examinations indicated that compared with group I control rats (Figure 1A,B), the media of the carotid arteries appeared thicker and SM cells had proliferated and become disorganized after LPS infusion into the adventitia in group II LPS-treated rats (Figure 1C,D). Morphological alterations were limited to the media of carotid arteries, as no obvious morphological alterations were observed in the adventitia and intima of the carotid arteries in group II LPS-treated rats (Figure 1C,D). Importantly, the proliferation and disorganization of SM cells induced by LPS infusion into the adventitia was completely reversed by pretreatment with the Rho-kinase inhibitor fasudil in group III fasudil + LPS-treated rats (Figure 1E,F) or by treatment with the cholesterol-lowering agent atorvastatin in group IV atorvastatin + LPS-treated rats (Figure 1G,H). Quantitative measurement of intima and media thickness showed that LPS significantly increased medial thickness, which was completely blocked by fasudil or atorvastatin (Table 1). Real-time RT-PCR demonstrated that LPS and pretreatment with fasudil or atorvastatin had an obvious effect on TNFα or IL-6 mRNA levels (Figure 1I,J,K,L).
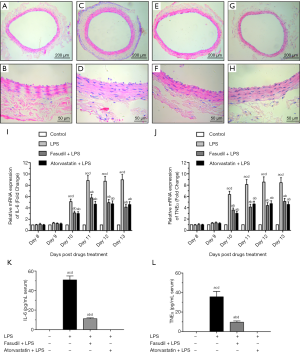

Full table
Adventitial LPS infusion had no obvious effect on the resting carotid blood flow rate when compared with the flow rate in the control rats (Table 2). Furthermore, fasudil and atorvastatin showed no apparent effect on resting carotid blood flow rate compared with the rate in the LPS-treated and control rats (Table 2). To evaluate the contractile responses of LPS-infused arteries, 1% serotonin was applied. Application of serotonin decreased carotid blood flow in control and LPS-treated rats, and the reduction induced by serotonin administration was significantly exacerbated in the LPS-treated rats compared with the control rats (Table 3), indicating that adventitial LPS infusion resulted in vascular hypersensitivity to serotonin. Fasudil completely blocked LPS-induced vessel hypersensitivity to serotonin (Table 3). Atorvastatin not only blocked the LPS-induced hypersensitivity, but it even increased carotid blood flow upon exposure to serotonin (Table 3).
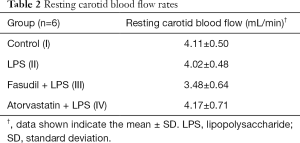
Full table
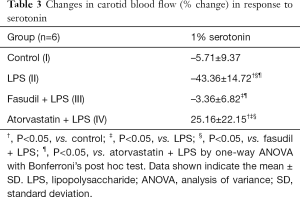
Full table
Adventitial LPS infusion caused a significant increase in ROCK2 protein expression (Figure 2A). To assess ROCK2 activity, phosphorylation of its substrate MYPT1 was analyzed. LPS significantly increased levels of phosphorylated MYPT1 protein (pMYPT1) and phosphorylated MLC (pMLC) without obviously affecting their total protein expression levels (Figure 2A). Both fasudil and atorvastatin significantly blocked the LPS-induced increase in ROCK2, pMYPT1 and pMLC levels (Figure 2A). Fasudil showed a stronger suppressive effect than atorvastatin on the LPS-induced increase in ROCK2 levels (Figure 2A). Neither fasudil nor atorvastatin showed obvious effects on total MYPT1 and MLC protein expression levels (Figure 2A). ROCK2 kinase activity in the pooled carotid arteries was also quantitatively measured. Consistent with increase in ROCK2 expression and phosphorylation of MYPT1, ROCK2 activity was significantly increased in LPS-treated group, which was suppressed by both fasudil and atorvastatin (Figure 2B). RT-PCR results indicated that neither LPS alone nor pretreatment with fasudil or atorvastatin had an obvious effect on ROCK2 or MYPT1 mRNA levels (Figure 2C). Western Blot results indicated that both LPS obviously inhibited the expressions of SERCA2α and SERCA2β, which could be restored by fasudil and atorvastatin (Figure 2D,E).
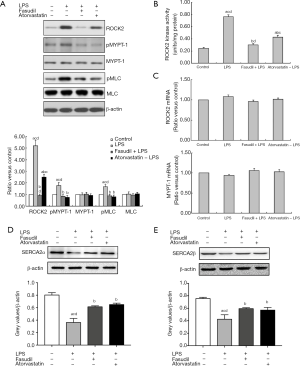
Adventitial LPS infusion significantly increased myocardin and SM α-actin protein expression (Figure 3A). LPS-induced increases in myocardin and SM α-actin levels were significantly suppressed by either fasudil or atorvastatin (Figure 3A). qPCR results indicated that LPS obviously increased mRNA expressions of SM α-actin and myocardin (Figure 3B), which could be restored by fasudil and atorvastatin.

Discussion
Many studies have suggested that the adventitia is activated during the development of atherosclerosis and hypertension, indicating a role for adventitia in providing cells and molecules with the capacity to influence neointima formation and vascular remodeling (17,20-23). The adventitia is also increasingly considered to be a highly active segment of vascular tissue that contributes to various disease-associated pathologies. Here, we demonstrated that transient LPS infusion to the adventitia of the rat common carotid artery induced vascular hypersensitivity to serotonin 5 days after surgery. Hypersensitivity to serotonin has been described in animal models of atherosclerosis and in patients with coronary atherosclerosis before the development of angiographically detectable atherosclerotic lesions (24-27). One of the most important steps for vascular SM contraction is phosphorylation of the MLC. The extent of MLC phosphorylation is ascribed to the balance between the activities of calcium/calmodulin-dependent MLC kinase and MLC phosphatase (MLCP). Rho-kinase, an effector of RhoA, has been shown to inhibit MLCP activity via the phosphorylation of its myosin-binding subunit, MYPT1, at Thr696. Thus, Rho-kinase plays a critical role in agonist-induced calcium sensitization and hypercontraction of VSMCs. We found that transient LPS infusion led to a significant increase in ROCK2 protein expression and phosphorylated MYPT1 levels. Direct measurement of ROCK2 activity confirmed that LPS increased ROCK2 activity of carotid arteries, while both atorvastatin and fasudil significantly blocked the effect. Importantly, the Rho-kinase inhibitor fasudil restored the hypercontraction of carotid arteries induced by LPS, implying that the activation of the RhoA/Rho-kinase signaling pathway plays an important role in LPS-induced alteration of vascular contractility. We also found that atorvastatin increased blood flow in the carotid artery, even after serotonin stimulation, indicating the potential for atorvastatin to combat coronary spasms. The mechanism by which atorvastatin prevents vascular spasm remains unclear. Statins are a class of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, and accumulating evidence supports the idea that they exert beneficial vascular effects that are independent of their cholesterol-lowering properties (28-31). Inhibition of ROCKs has recently been ascribed to the beneficial effects of statins on the cardiovascular system (28). We demonstrated that fasudil and atorvastatin had similar magnitudes of suppressive effects on LPS-induced phosphorylation of MYPT1 and activation of ROCK2, although atorvastatin showed weaker inhibitory effects on ROCK2 expression than fasudil. Atorvastatin might suppress ROCK2 activity without causing a profound reduction in its expression. Alternatively, atorvastatin might inhibit other kinase(s) responsible for the phosphorylation of MYPT1. The mechanism underlying the inhibition of MYPT1 phosphorylation and ROCK2 activation by atorvastatin will require further investigation in the future.
The arterial wall represents a highly plastic three-dimensional structure with a unique capacity to adapt to local changes during development and in some pathological vascular conditions (32-35). Throughout embryonic development VSMCs proliferate and mature into a differentiated contractile state. Postnatally, VSMCs remain in the differentiated state and express stereotypical markers, such as myocardin, SM-MHC, calponin, and SM22α, and remain quiescent until injury or disease-associated pathologies, such as atherosclerosis, promote the phenotypic transition from a differentiated to a proliferative state. We demonstrated that LPS-induced transient adventitial inflammation resulted in the proliferation and disordered arrangement of VSMCs in the medial layer, whereas no obvious alterations in the intimal or adventitial layers were observed.
Inconsistence with our observations, it has been reported that LPS promotes proliferation and migration of cultured rat adventitial fibroblast cells (36), and LPS results in vascular hyporeactivity to noradrenalin in cultured rat carotid arteries (37). The current study investigated the influence of LPS on carotid arteries in vivo, while the previous experiments investigated the effect of LPS on cultured adventitial fibroblast (36) or cultured fragments of vessels (37). The differences between in vivo and in vitro systems used in these studies might ascribe to the apparent different phenomena. Alternatively, the exposure period may also contribute to the difference. The previous studies examined the influence of LPS within 24 h, while the current study was performed at 5 days after LPS exposure. It should be noted that 5 days after LPS infusion might not allow sufficient time for intimal thickening to occur, because this would be predicted to occur after the infiltration of inflammatory cells diminishes and tissue repair has occurred. Investigations at different time points upon LPS exposure are required in the future. myocardin has been shown to play a critical role during early SMC differentiation and to transactivate many SMC differentiation markers, such as SM α-actin, SM22, and SM-MHC (4,38,39). We demonstrated that transient LPS infusion caused protein levels of myocardin to increase, while myocardin mRNA expression levels were unaffected. Thus, LPS might induce myocardin expression at the post-transcriptional level. Consistent with the LPS-induced increase in myocardin protein expression levels, SM α-actin levels were also significantly increased.
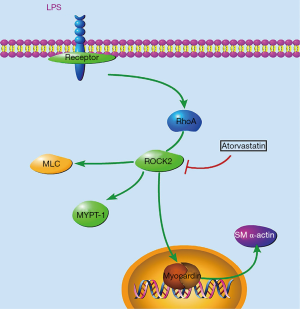
In summary, we demonstrated that the transient addition of LPS to the adventitia caused vascular hypersensitivity and remodeling via the RhoA/Rho-kinase signaling pathway, accompanied by the dysregulation of some stereotypical VSMC genes. Additionally, we found that similar to the Rho-kinase inhibitor fasudil, the cholesterol-lowering agent atorvastatin showed beneficial effects on vascular dysfunction mediated by LPS via suppression of RhoA/Rho-kinase signaling pathway (as shown in Figure 4). To our knowledge, the current study for the first time demonstrated that transient exposure of adventitia to LPS resulted in chronic alterations in vascular functions and VSMCs in vivo, reflecting the critical role of adventitia in the development of vascular diseases.
Acknowledgments
Funding: This work was supported by a grant from the National Basic Research Program (973 Program) of China to DY Zeng (2005CB523310).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal protocols were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the China Medical University Animal Care and Use Committee (No. 2018157).
References
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767-801. [Crossref] [PubMed]
- Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg 2007;45 Suppl A:A25-32.
- Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 2003;35:577-93. [Crossref] [PubMed]
- Du KL, Ip HS, Li J, et al. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 2003;23:2425-37. [Crossref] [PubMed]
- Chen J, Kitchen CM, Streb JW, et al. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 2002;34:1345-56. [Crossref] [PubMed]
- Sun Q, Taurin S, Sethakorn N, et al. Myocardin-dependent activation of the CArG box-rich smooth muscle gamma-actin gene: preferential utilization of a single CArG element through functional association with the NKX3.1 homeodomain protein. J Biol Chem 2009;284:32582-90. [Crossref] [PubMed]
- Doi H, Iso T, Yamazaki M, et al. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler Thromb Vasc Biol 2005;25:2328-34. [Crossref] [PubMed]
- Speer MY, Yang HY, Brabb T, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res 2009;104:733-41. [Crossref] [PubMed]
- Chow N, Bell RD, Deane R, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A 2007;104:823-8. [Crossref] [PubMed]
- Renaudin A, Lehmann M, Girault J, et al. Organization of point contacts in neuronal growth cones. J Neurosci Res 1999;55:458-71. [Crossref] [PubMed]
- Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 2004;301:43-9. [Crossref] [PubMed]
- Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett 2001;165:1-10. [Crossref] [PubMed]
- Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 2006;290:C661-8. [Crossref] [PubMed]
- Wamhoff BR, Bowles DK, McDonald OG, et al. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res 2004;95:406-14. [Crossref] [PubMed]
- Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev 2006;20:1545-56. [Crossref] [PubMed]
- Muscoli C, Cuzzocrea S, Riley DP, et al. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol 2003;140:445-60. [Crossref] [PubMed]
- Wilcox JN, Scott NA. Potential role of the adventitia in arteritis and atherosclerosis. Int J Cardiol 1996;54 Suppl:S21-35. [Crossref] [PubMed]
- Piñeiro R, Iglesias MJ, Gallego R, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 2005;579:5163-9. [Crossref] [PubMed]
- Tan KC, Xu A, Chow WS, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab 2004;89:765-9. [Crossref] [PubMed]
- Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol 1997;17:417-22. [Crossref] [PubMed]
- Faggin E, Puato M, Zardo L, et al. Smooth muscle-specific SM22 protein is expressed in the adventitial cells of balloon-injured rabbit carotid artery. Arterioscler Thromb Vasc Biol 1999;19:1393-404. [Crossref] [PubMed]
- Li G, Chen SJ, Oparil S, et al. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation 2000;101:1362-5. [Crossref] [PubMed]
- Gutterman DD. Adventitia-dependent influences on vascular function. Am J Physiol 1999;277:H1265-72. [PubMed]
- Villa AE, Guzman LA, Chen W, et al. Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. J Clin Invest 1994;93:1243-9. [Crossref] [PubMed]
- Vrints CJ, Bult H, Bosmans J, et al. Paradoxical vasoconstriction as result of acetylcholine and serotonin in diseased human coronary arteries. Eur Heart J 1992;13:824-31. [Crossref] [PubMed]
- De Meyer GR, Bult H, Martin JF, et al. The effect of a developing neo-intima on serotonergic and adrenergic contractions. Eur J Pharmacol 1990;187:519-24. [Crossref] [PubMed]
- Van Put DJ, Van Hove CE, De Meyer GR, et al. Dexamethasone influences intimal thickening and vascular reactivity in the rabbit collared carotid artery. Eur J Pharmacol 1995;294:753-61. [Crossref] [PubMed]
- Wang L, Zhang X, Liu L, et al. Atorvastatin protects rat brains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors (RAGE and TLR4), NF-kappaB expression. Neurosci Lett 2010;471:152-6. [Crossref] [PubMed]
- Inoue I, Goto S, Mizotani K, et al. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci 2000;67:863-76. [Crossref] [PubMed]
- Wang H, Lynch JR, Song P, et al. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol 2007;206:59-69. [Crossref] [PubMed]
- McGirt MJ, Lynch JR, Parra A, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke 2002;33:2950-6. [Crossref] [PubMed]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 1995;75:487-517. [Crossref] [PubMed]
- Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 1997;59:89-144. [Crossref] [PubMed]
- Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res 1999;36:2-27. [Crossref] [PubMed]
- Cai J, Kirlin WG, Chen Y, et al. Overexpression of heat shock factor 1 inhibits butyrate-induced differentiation in colon cancer cells. Cell Stress Chaperones 2006;11:199-207. [Crossref] [PubMed]
- Xiao-jun C, Min F, Liang C, et al. Expression and role of adiponectin receptor 1 in lipopolysaccharide-induced proliferation of cultured rat adventitial fibroblasts. Cell Biol Int 2010;34:163-9. [Crossref] [PubMed]
- Kleschyov AL, Muller B, Schott C, et al. Role of adventitial nitric oxide in vascular hyporeactivity induced by lipopolysaccharide in rat aorta. Br J Pharmacol 1998;124:623-6. [Crossref] [PubMed]
- Yoshida T, Sinha S, Dandré F, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 2003;92:856-64. [Crossref] [PubMed]
- Li S, Wang DZ, Wang Z, et al. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A 2003;100:9366-70. [Crossref] [PubMed]


