
A simplified new-generation renal mass complexity scoring system
Partial nephrectomy is the preferred surgical approach to localized renal tumors as this technique offers favorable oncologic outcomes and maximizes long-term renal function (1,2). Ten years ago, the first wave of standardized renal mass scoring nomograms was introduced to help clinicians objectively stratify the complexity of localized renal tumors and improve operative decision-making (3,4). Since that time, multiple series have validated the use of nomograms in predicting perioperative complications and outcomes following surgery and ablative procedures (5,6). Nephrometry scores have also proven to be useful when comparing partial nephrectomy series from different institutions and when counselling patients regarding their individualized surgical risks (7). However, concerns have been raised regarding reproducibility and interobserver variability of the first-generation nephrometry systems (8). Their clinical usefulness has also been questioned as certain nephrometry calculations can be time-consuming and are unlikely to alter the course of treatment in an experienced surgeon’s practice (9).
Ficarra and colleagues recognized these limitations and planned to address them by constructing a new, simplified nephrometry scoring system. In this multi-institutional retrospective study, the authors aimed to condense the existing preoperative aspects and dimensions used for an anatomical (PADUA) model by testing the strength of association of each of its variables to overall post-operative complication rates. Using preoperative cross-sectional imaging, each tumor in a cohort of 531 patients who underwent partial nephrectomy was assigned a standard PADUA score based on six anatomic variables. Contact surface area (CSA) was also calculated based on tumor size and degree of intraparenchymal extension (10). Binary logistic regression analysis was used to determine which variables carried the most clinical significance and receiver-operating curve (ROC) analysis was used to compare the new nephrometry system to the original version.
In this series, the authors found that only four of the six variables of the PADUA score were required to accurately predict the overall post-operative complication rate. The most important anatomic features were found to be (I) rim location; (II) renal sinus involvement; (III) exophytic rate; (IV) maximal tumor diameter. These variables were used to piece together a new Simplified PAdua REnal (SPARE) system, which was ultimately found to perform similar to the original PADUA model using ROC analysis. Urinary collecting system (UCS) involvement and polar location (upper/mid/lower) were found to have weaker predictive value and were omitted from the newer SPARE model. Higher SPARE scores not only predicted higher rates of overall complications (low-risk 18.4% versus high-risk 48.6%), but also correlated with prolonged operative times, longer warm ischemia times, and higher levels of estimated blood loss. SPARE scores were not useful in predicting post-operative renal function. The CSA parameter did not improve the accuracy of either nephrometry system, but was found to be an independent predictor of renal function three months after surgery.
In the current literature, at least 13 different nephrometry scoring systems exist (Table 1) and greater than 100 studies have focused on their validation, applicability, and predictive value. However, the clinical importance of renal mass scoring systems is often criticized due to subjective aspects in each grading system, limited benefit in predicting histology/grade and post-operative renal function, and impractical use in a busy clinical setting (20,21). It is not surprising that in the SPARE study, like many similar studies, maximal tumor diameter was found to be one of the strongest predictors of post-operative complications. However, in this series the authors pointed out that “size alone” was inferior to the PADUA and SPARE systems in predicting post-operative complications. Other authors have reported contradictory findings. Maximal tumor diameter alone has been shown in some series to perform better than RENAL and PADUA in predicting complications and recurrence following ablative procedures (22,23). Given that renal mass diameter is a common denominator amongst nearly all the nephrometry scoring systems and has consistently proven its predictive power (24), we must ask ourselves, what are we gaining by adding and subtracting other variables to create a longer list of available nomograms? Could we simply use tumor size and clinical intuition to drive our surgical decision-making? Is maximal tumor diameter alone sufficient for comparing series of patients undergoing nephron sparing procedures and comparing associated oncologic and functional outcomes?
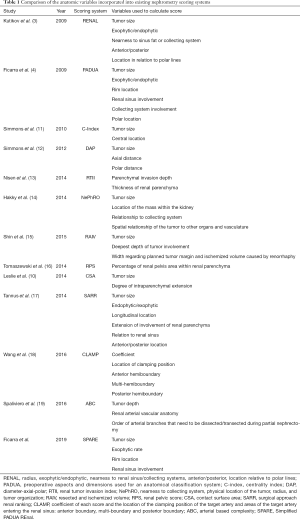
Full table
When looking back at the original PADUA study, which was a prospective trial at a single institution consisting of 164 patients who all underwent open extraperitoneal partial nephrectomy without vessel clamping, the rate of overall post-operative complications was correlated with polar location, rim location, involvement of the sinus, involvement of the UCS, and percentage of tumor extension into the kidney (4). In the current SPARE study, which incorporates a larger retrospective cohort of 531 patients from multiple centers who underwent open (44.6%), pure laparoscopic (28.6%), or robot-assisted (26.7%) partial nephrectomy, polar location and UCS involvement were no longer found to be significant. This finding may be explained by the discrepancy in the maximal diameter of the tumors in each study, as the total number of larger renal masses measuring 4–7 cm (pT1b) was lower in the PADUA trial (11.8%) compared to the SPARE study (23.9%) (4). This reinforces the theory that as tumor diameter increases, the less additional variables are needed to predict post-operative complications. Still, selection bias and series heterogeneity are inherent to the various nephrometry scoring studies and likely contribute to their inconsistent findings, such as the differences reported in these two series by the same author.
The authors are commended for recognizing the limitations that exist in the current space of nephrometry nomograms and for offering a simpler approach to stratifying renal tumors. Perhaps by requiring the entry of two less variables, the SPARE model will be appreciated by current PADUA users. Perhaps SPARE’s ease of use will entice a new generation of surgeons to incorporate nomograms into their daily practice. It will be interesting to see if SPARE will be externally validated and how it will compare to the other existing nephrometry systems in future series.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol 2017;198:520-9. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Kidney Cancer (Version 1.2020). Accessed July 15, 2019. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844-53. [Crossref] [PubMed]
- Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 2009;56:786-93. [Crossref] [PubMed]
- Chang X, Ji C, Zhao X, et al. The application of R.E.N.A.L. nephrometry scoring system in predicting the complications after laparoscopic renal radiofrequency ablation. J Endourol 2014;28:424-9. [Crossref] [PubMed]
- Simhan J, Smaldone MC, Tsai KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol 2011;60:724-30. [Crossref] [PubMed]
- Canter D, Kutikov A, Manley B, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology 2011;78:1089-94. [Crossref] [PubMed]
- Spaliviero M, Poon BY, Aras O, et al. Interobserver variability of R.E.N.A.L., PADUA, and centrality index nephrometry score systems. World J Urol 2015;33:853-8. [Crossref] [PubMed]
- Sharma P, McCormick BZ, Zargar-Shoshtari K, et al. Is surgeon intuition equivalent to models of operative complexity in determining the surgical approach for nephron sparing surgery? Indian J Urol 2016;32:124-31. [Crossref] [PubMed]
- Leslie S, Gill IS, de Castro Abreu AL, et al. Renal tumor contact surface area: a novel parameter for predicting complexity and outcomes of partial nephrectomy. Eur Urol 2014;66:884-93. [Crossref] [PubMed]
- Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method. J Urol 2010;183:1708-13. [Crossref] [PubMed]
- Simmons MN, Hillyer SP, Lee BH, et al. Diameter-axial-polar nephrometry: integration and optimization of R.E.N.A.L. and centrality index scoring systems. J Urol 2012;188:384-90. [Crossref] [PubMed]
- Nisen H, Ruutu M, Glucker E, et al. Renal tumour invasion index as a novel anatomical classification predicting urological complications after partial nephrectomy. Scand J Urol 2014;48:41-51. [Crossref] [PubMed]
- Hakky TS, Baumgarten AS, Allen B, et al. Zonal NePhRO scoring system: a superior renal tumor complexity classification model. Clin Genitourin Cancer 2014;12:e13-8. [Crossref] [PubMed]
- Shin TY, Komninos C, Kim DW, et al. A novel mathematical model to predict the severity of postoperative functional reduction before partial nephrectomy: the importance of calculating resected and ischemic volume. J Urol 2015;193:423-9. [Crossref] [PubMed]
- Tomaszewski JJ, Cung B, Smaldone MC, et al. Renal pelvic anatomy is associated with incidence, grade, and need for intervention for urine leak following partial nephrectomy. Eur Urol 2014;66:949-55. [Crossref] [PubMed]
- Tannus M, Goldman SM, Andreoni C. Practical and intuitive surgical approach renal ranking to predict outcomes in the management of renal tumors: a novel score tool. J Endourol 2014;28:487-92. [Crossref] [PubMed]
- Wang HK, Qin XJ, Ma CG, et al. Nephrometry score-guided off-clamp laparoscopic partial nephrectomy: patient selection and short-time functional results. World J Surg Oncol 2016;14:163. [Crossref] [PubMed]
- Spaliviero M, Poon BY, Karlo CA, et al. An Arterial Based Complexity (ABC) Scoring System to Assess the Morbidity Profile of Partial Nephrectomy. Eur Urol 2016;69:72-9. [Crossref] [PubMed]
- Chapin BF, Wood CG. The RENAL nephrometry nomogram: statistically significant, but is it clinically relevant? Eur Urol 2011;60:249-51; discussion 251-2. [Crossref] [PubMed]
- Buethe DD, Moussly S, Lin HY, et al. Is the R.E.N.A.L. nephrometry scoring system predictive of the functional efficacy of nephron sparing surgery in the solitary kidney? J Urol 2012;188:729-35. [Crossref] [PubMed]
- Seideman CA, Gahan J, Weaver M, et al. Renal tumour nephrometry score does not correlate with the risk of radiofrequency ablation complications. BJU Int 2013;112:1121-4. [Crossref] [PubMed]
- Maxwell AWP, Baird GL, Iannuccilli JD, et al. Renal Cell Carcinoma: Comparison of RENAL Nephrometry and PADUA Scores with Maximum Tumor Diameter for Prediction of Local Recurrence after Thermal Ablation. Radiology 2017;283:590-7. [Crossref] [PubMed]
- Tsivian M, Mouraviev V, Albala DM, et al. Clinical predictors of renal mass pathological features. BJU Int 2011;107:735-40. [Crossref] [PubMed]


