
Do we have a “game changer” in treating patients with brain metastasis from renal cell carcinoma?
Renal cell carcinoma (RCC) is the most common type of kidney cancer. About 25% of patients are diagnosed with metastatic disease at presentation and another 25–30% diagnosed with localized disease will develop metastasis over time (1). The incidence of brain metastasis (BM) ranged from 3–17% in various studies. Historically, the prognosis of patients with brain metastasis was very poor, with a median survival of up to seven months in more contemporary series (2). With targeted therapy, according to the International Metastatic RCC Database Consortium (IMDC) [2005–2011], median survival time has improved, reaching 14.4 months after first-line treatment for patients with brain metastasis vs. 19.0 months for those with no brain metastasis (3). The prognosis and preferred treatment strategy for patients with brain metastases depends on several factors, including: number, size and location of brain metastases, neurologic symptoms, performance status, tumor histology, extracranial disease status, previous systemic treatments and additional available treatment options (4,5).
Local therapy remains the most effective type of treatment, including surgery, stereotactic radiosurgery (SRS) and whole brain radiotherapy (WBRT), or a combination of surgery and radiation.
Surgery as a single modality treatment may give good results with immediate relief of mass-effect related symptoms. However, rates of local failure are relatively high, up to 40% (6,7). The use of surgery in RCC BM is limited to fit patients, usually at a younger age, less symptomatic, with a Karnofsky Performance Status (KPS) of more than 80, and most importantly, with no more than three metastases. Although there are reports of resection of multiple brain metastasis (8), most of the data available is for patients with one resectable brain metastasis (9). Median survival of patients after craniotomy ranges between 8.5–12.6 months in various reports (4,7,8).
In the past decade, SRS has become the treatment of choice for patients who are not surgical candidates, especially those with multiple metastases (up to 10) (10). This type of treatment may provide 1 year local control rates of up to 90–95% (4,10), with median OS rates of 13.9 months from diagnosis of BM (5,10). Median reported total dose for SRS ranges between 20–22 Gy in different reports, delivered in 1–5 fractions (4). Recommendations of SRS single fraction treatments of maximum doses of 24, 18, and 15 Gy to tumors of 2 cm, between 2–3 cm, and greater than 3 cm, respectively, were given in the RTOG criteria (11,12). Using a dose of 20 Gy in SRS treatment compared to a dose of 16–18 Gy demonstrated better local control rates (12-month local control rates of 81% and 50%, respectively; P=0.001) (4).
This treatment option has a number of advantages over surgery, including a higher number of metastases that may be treated at the same time, lower rate of neurological complications, and the opportunity to treat brain metastases in areas not fit for surgery. The main limitations of this type of therapy are the high rates of intracranial failures, up to 50% (13).
WBRT is another treatment option for patients with BM, but it has several limitations as a single treatment modality in brain metastases from RCC. This tumor type is considered radio-resistant, requiring high doses of radiation for good local control that cannot be delivered to the whole brain. Standard doses usually used for WBRT are not always effective in patients with BM from RCC, leading to a very short median OS, about 4.4 months (14). It remains as the preferred choice for patients with brain metastases not amenable to surgery or SRS, especially for patients with multiple BM (more than 10), poorly controlled systemic disease and a relatively short life expectancy. The standard dose recommended for WBRT in RCC patients with multiple brain metastases is 30 Gy delivered in 10 fractions; dose escalation regimens did not succeed in improving OS in this group of patients in almost all prospective trials (15). Rades et al. performed a retrospective analysis of treatment outcomes in 60 RCC patients with BM, treated with WBRT, comparing higher doses (40 Gy in 20 fractions or 45 Gy in 15 fractions) with standard treatment regimens. Higher doses treated patients had a median OS of 1 year and local control rates of 57% for 6 months, compared to lower doses treated patients, with 4 months median OS and local control rates at 6 months of 21% only (15). One of the main limitations of WBRT, especially in high doses, is cognitive impairment. New techniques with sparing of the hippocampal structures showed less cognitive injury, with similar general treatment effectiveness (16).
When considering local therapy, a combined approach may be more effective in all points of view. It includes a combination of surgery with SRS for better local control, or surgery or SRS with WBRT for better intracranial disease control.
In a phase III randomized trial carried out 20 years ago, the benefit of postoperative WBRT was a 52% reduction in intracranial recurrences (17). More recent retrospective studies supported the point of combined surgery and adjuvant WBRT to improve survival in patients without evidence of extracranial disease but not in patients with uncontrolled systemic disease (14).
A consecutive series of SRS alone, surgery plus SRS, and WBRT plus SRS demonstrated overall survival times of 13.9, 21.9, and 5.9 months, respectively, with local control rates of 84%, 94%, and 88%, respectively (4).
In another trial with 88 patients evaluating the role of SRS and WBRT in brain metastases from RCC, the median OS for SRS only, SRS and WBRT or WBRT only was 12, 16, and 2 months, respectively (18). Although RCC is considered to be a radio-resistant tumor, WBRT might affect microscopic metastases and potential delay in the appearance of new brain metastases. Nevertheless, no significant survival benefit could be demonstrated in these patients. A selection bias might partially explain this result, while WBRT alone was frequently used in patients with a larger number of brain metastases (4).
According to the reported data, aggressive treatment with combined techniques is recommended for local treatment of patients with brain metastases from RCC, including a combination of surgery with adjuvant SRS. In some cases, adding WBI is justified. Table 1 summarizes the data on local treatments for brain metastasis from RCC.
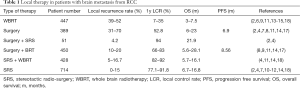
Full table
There is restricted data considering the activity of systemic agents in brain metastases from RCC. From historical treatment options, high-dose interleukin-2 showed overall response rates (ORR) of 5.6%, with the addition 22.3% of the patients with disease stabilization in a group of 18 patients. The median interval between the diagnosis of brain metastases and the beginning of IL-2 therapy was 7.0 months (range, 1–116 months). Median overall survival in patients with brain metastases was 15.3 months, compared to 48 months in RCC patients without any history of brain metastases (19).
Tyrosine kinase inhibitors (TKIs) are small molecules able to cross the blood-brain barrier, but their therapeutic activity on BM is limited due to the presence of drug efflux transporters. Table 2 summarizes the data on the effectiveness of TKIs in patients with brain metastases from RCC.
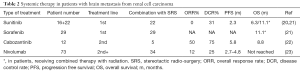
Full table
There are two reports of sunitinib activity in patients with BM. Analysis of an open access trial that included 321 patients with brain metastases treated with sunitinib in standard doses demonstrated that 213 (66%) of them were evaluable for tumor response. Complete response was reported for one patient while 25 patients (12%) had a partial response with ORR of 12%, in contrast to 17% ORR in the whole study population. Of the 213 evaluable patients, stable disease was reported for 111 patients (52%) for at least 3 months, following with a clinical benefit rate of 64%. Patients with brain metastases showed a median PFS of 5.6 months, whereas median PFS was 10.9 months in the overall extended access program (EAP) population. Median OS was 9.2 months (95% CI, 7.8–10.9) in patients with brain metastases, contrasting to 18.4 months (95% CI, 17.4–19.2) in the overall EAP population (24). Based on these results, a prospective phase II trial was conducted to better evaluate efficacy of sunitinib in previously untreated patients. Exclusion criteria included cerebral metastasis presenting as hemorrhage, presence of an isolated BM of less than 2 cm amenable to surgery or radiosurgery. The study was stopped after accrual of 17 patients. No objective responses were reported. Best response was disease stabilization, seen in 5 patients (31%). Median TTP was 2.3 months and median OS was 6.3 months (95% CI, 2.1–7.9 months) (20).
Another study based on real life data analyzed the activity of cabozantinib as a second or more treatment line in patients with newly presented BM from RCC. The analysis was made on 12 patients who presented with BM; most had a single lesion (75%), and all patients were initially or simultaneously treated with local treatment (surgery, SRS or WBRT). Overall response rate was 50%, with a disease control rate of 75%, evaluated according to RECIST criteria. The median duration of response was 4.8 months and median OS was 8.8 months, with a 1 year OS of 50% (22).
A study of combined local and systemic therapy reported efficacy and safety of antiangiogenic agents (sunitinib and sorafenib) with concomitant SRS in patients with cerebral and spinal metastasis from RCC. Of a total of 51 patients with BM, 22 were treated with sunitinib and 29 with sorafenib. Most had been treated with single fraction SRS in a dose of 20 Gy for a median of 2.6 lesions. Local control of cerebral lesions at 12 and 24 months was 100% and 96.6% (SEM 0.03, 95% CI, 78–99%), respectively. Median overall survival of 11 months from SRS was shown in patients with cerebral lesions. Following 36 months from SRS, 25% of the patients were still alive (21). This study supports the approach of combined therapy, with aggressive local treatment added to systemic treatment. Despite the small sample, there is a meaningful difference in survival and local control in these patients, including those on second line systemic therapy.
In recent years, immunotherapy has been showing promising activity in patients with metastatic RCC. There is limited data concerning the immune structure of brain metastases from RCC. These lesions are frequently characterized by lymphocytic infiltration and programmed death-ligand 1 (PD-L1) expression, given the rationale of checkpoint inhibitor evaluation in patients with BM (23).
The GETUG-AFU 26 NIVOREN phase II trial assessed the activity and safety of nivolumab in patients with metastatic clear cell RCC to the brain who failed vascular endothelial growth factor–directed therapies. The rate of overall survival at 1 year was 66.7% (95% CI, 49.6–79.1%) in patients without local therapy (cohort A), and 58.8% (95% CI, 40.6–73.2%) in those previously treated locally (cohort B). Median intracranial PFS was 2.7 months (95% CI, 2.3–4.6 months) in cohort A and 4.8 months (95% CI, 3.0–8.0) in cohort B. The intracranial activity of nivolumab shown in this study in patients with untreated brain metastases from RCC was very limited. Only four patients (12%) out of 34 had intracranial response. In addition, all the responding patients had limited intracranial tumor burden (up to 10 mm). Actually, the reduction in the risk of intracranial progression was shown only for patients who had received prior focal therapy, compared to those with untreated brain metastases (23) (Table 2).
Checkpoint combinations may show better results, according to their activity in a general population of patients with metastatic RCC, with intermediate and poor IMDC risk groups. This hypothesis is supported by the results of phase II trials in melanoma. The combination of nivolumab with ipilimumab supplied high intracranial response rates among 94 patients with metastatic melanoma to the brain. Complete response rate was 26% and the partial response rate was 30% for a total of 57% intracranial clinical benefit for those patients (25). Combinations of antiangiogenics and immune checkpoint inhibitors may provide improved antitumor immunity in the brain that may deplete myeloid-derived suppressor cells, allowing conversion of “cold tumors” into “hot tumors”. Most probably, these combinations will soon emerge as first-line standard treatment for patients with metastatic RCC, and consecrated trials are awaited to evaluate their impact on patients with BM.
To conclude: local treatment options remain the most important treatment modality of patients with brain metastasis from RCC. Systemic therapy as a single modality is not effective enough. Further dedicated trials with combined therapies of two checkpoint inhibitors or one drug with TKIs are needed to estimate their impact on outcomes in these patients.
The best treatment strategy for patients with brain metastases from clear cell RCC is a combination of local aggressive therapy (surgery, SRS or combination) with the optional use of WBRT, preferably with hippocampal sparing techniques and effective systemic therapy, including second line treatment options (TKI or immunotherapy or combinations).
For each treatment approach, proper patient selection is needed. In patients with poor prognosis or low KPS, uncontrolled primary and lack of further systemic treatment options—a less aggressive approach may be acceptable.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843-52. [Crossref] [PubMed]
- Shuch B, La Rochelle JC, Klatte T, et al. Brain metastasis from renal cell carcinoma: presentation, recurrence, and survival. Cancer 2008;113:1641-8. [Crossref] [PubMed]
- Vickers MM, Al-Harbi H, Choueiri TK, et al. Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy: results from the international metastatic renal cell carcinoma database consortium. Clin Genitourin Cancer 2013;11:311-5. [Crossref] [PubMed]
- Ippen FM, Mahadevan A, Wong ET, et al. Stereotactic radiosurgery for renal cancer brain metastasis: prognostic factors and the role of whole-brain radiation and surgical resection. J Oncol 2015;2015:636918. [Crossref] [PubMed]
- Choi SY, Yoo S, You D, et al. Prognostic factors for survival of patients with synchronous or metachronous brain metastasis of renal cell carcinoma. Clin Genitourin Cancer 2017;15:717-23. [Crossref] [PubMed]
- Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg 1993;79:210-6. [Crossref] [PubMed]
- Churilla TM, Chowdhury I, Handorf E, et al. Comparison of local control of brain metastasis with stereotactic radiosurgery versus surgical resection: a secondary analysis of EORTC 22952-26001. Int J Radiat Oncol 2017;99:S158-9. [Crossref]
- Sivasanker M, Madhugiri VS, Moiyadi AV, et al. Surgery for brain metastases: An analysis of outcomes and factors affecting survival. Clin Neurol Neurosurg 2018;168:153-62. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Wardak Z, Christie A, Bowman A, et al. Stereotactic radiosurgery for multiple brain metastases from renal-cell carcinoma. Clin Genitourin Cancer 2019;17:e273-80. [Crossref] [PubMed]
- Elaimy AL, Mackay AR, Lamoreaux WT, et al. Clinical outcomes of stereotactic radiosurgery in the treatment of patients with metastatic brain tumors. World Neurosurg 2011;75:673-83. [Crossref] [PubMed]
- Hanson PW, Elaimy AL, Lamoreaux WT, et al. A concise review of the efficacy of stereotactic radiosurgery in the management of melanoma and renal cell carcinoma brain metastases. World J Surg Oncol 2012;10:176. [Crossref] [PubMed]
- Wrónski M, Maor MH, Davis BJ, et al. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys 1997;37:753-9. [Crossref] [PubMed]
- Doh LS, Amato R, Paulino AC, et al. Radiation therapy in the management of brain metastases from renal cell carcinoma. Oncology 2006;20:603-13. [PubMed]
- Rades D, Heisterkamp C, Schild SE. Do patients receiving whole-brain radiotherapy for brain metastases from renal cell carcinoma benefit from escalation of the radiation dose? Int J Radiat Oncol Biol Phys 2010;78:398-403. [Crossref] [PubMed]
- Vrána D, Študentová H, Matzenauer M, et al. Treatment of brain metastases of renal cell cancer with combined hypofractionated stereotactic radiotherapy and whole brain radiotherapy with hippocampal sparing. Oncol Lett 2016;11:3777-81. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998;280:1485-9. [Crossref] [PubMed]
- Fokas E, Henzel M, Hamm K, et al. Radiotherapy for brain metastases from renal cell cancer: should whole-brain radiotherapy be added to stereotactic radiosurgery? analysis of 88 patients. Strahlenther Onkol 2010;186:210-7. [Crossref] [PubMed]
- Chandar A, Silk AW, Clark JI, et al. Efficacy and safety of high-dose interleukin-2 treatment in patients with a history of brain metastases from renal cell carcinoma. J Immunother Cancer 2015;3:129. [Crossref]
- Chevreau C, Ravaud A, Escudier B, et al. French Group on Renal Cancer. A phase II trial of sunitinib in patients with renal cell cancer and untreated brain metastases. Clin Genitourin Cancer 2014;12:50-4. [Crossref] [PubMed]
- Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int 2011;108:673-8. [PubMed]
- Peverelli G, Raimondi A, Ratta R, et al. Cabozantinib in renal cell carcinoma with brain metastases: safety and efficacy in a real-world population. Clin Genitourin Cancer 2019;17:291-8. [Crossref] [PubMed]
- Flippot R, Dalban C, Laguerre B, et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: Results of the GETUG-AFU 26 NIVOREN Multicenter Phase II Study. J Clin Oncol 2019;37:2008-16. [Crossref] [PubMed]
- Gore ME, Hariharan S, Porta C, et al. Sunitinib in metastatic renal cell carcinoma patients with brain metastases. Cancer 2011;117:501-9. [Crossref] [PubMed]
- Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018;379:722-30. [Crossref] [PubMed]


