Diagnostic value, safety, and histopathologic discrepancy risk factors for endoscopic forceps biopsy and transrectal ultrasound-guided core needle biopsy in rectum lesions
Introduction
Colorectal cancer is one of the most common malignancies worldwide, and its morbidity and mortality have been increasing over time in China (1,2). Accurate diagnosis of protruding lesions, especially in cancer in the rectum, is crucial for preoperative clinical staging and decision making for initial treatment; concerns and decisions include the extent of surgical resection (radical or palliative), surgical methods (such as transanal endoscopic microsurgery (3), endoscopic submucosal dissection, endoscopic mucosal resection or total mesorectal excision (4), and the use of preoperative chemotherapy/radiotherapy. The most common pathologic types of protruding lesions in the rectum include adenocarcinoma and adenoma, but other pathologic types [such as squamous cell carcinoma, mucinous adenocarcinoma, neuroendocrine cancer (5), and inflammatory polyps, etc.] have also been observed in patients. Although these other pathologic types have a low incidence, their treatments and prognosis are significantly different. Thus, preoperative pathologic diagnosis is very important.
The 2018 National Comprehensive Cancer Network Guideline (6) recommends biopsy and pathologic examination in clinical examinations of rectal cancer or adenoma with suspected invasive cancer. Endoscopic forceps biopsy (EFB) is an important technique for the early diagnosis of rectal lesions, but it is usually limited for technical reasons, or is insufficient or unsatisfactory for diagnosis (7). Thus, in some cases, invasion of submucosa (ISM) cannot be confirmed in tissues collected by EFB although the latter may indicate histologic characteristics of rectal cancer. According to the WHO criteria (2000), these cases can be diagnosed only with high-grade intraepithelial neoplasia (HGIN). HGIN is characterized by a lack of evidence of submucosal invasion and a morphologically characteristic cancer (8). However, postoperative pathology reveals that most such lesions are invasive cancers with ISM or deeper layers (7,9-11). Evidence shows that invasive cancers can be confirmed only in 58.7–67.3% of rectal tissues collected by EFB before surgery (12,13). Thus, EFB cannot be used as the only standard in the preoperative diagnosis of rectal lesions, as its exclusive use may cause significant underdiagnosis and treatment delay (7,9-11,14,15).
To overcome these limitations, transrectal ultrasound (TRUS)-guided core needle biopsy (CNB) has been developed. TRUS can be used for evaluation, and biopsy can be performed under the guidance of intraoperative ultrasound. In this manner, the samples collected are not confined to the superficial tissues, and the five-layer structure of the rectal wall can be harvested for further examination. A multicenter study found CNB has a high accuracy and safety in the diagnosis of gastrointestinal subepithelial lesions (sensitivity 85%, specificity 100%) (16). For these reasons, TRUS-guided CNB is an effective method for the initial biopsy diagnosis of rectal lesions.
At present, little is known about the risk factors for histopathologic discrepancies of these two biopsy methods, nor is much clear concerning the diagnostic value and safety of TRUS-guided CNB after EFB diagnosis of rectal lesions. Consequently, this study aimed (I) to determine the diagnostic value and safety of EFB and TRUS-guided CNB, and (II) to analyze the risk factors of their histopathologic discrepancies, with a particular focus in identifying the indicators for re-biopsy using TRUS-guided CNB after EFB.
Methods
Patients
From October 2017 and January 2019, 132 consecutive patients with diagnoses of rectal lesions who received initial EFB and TRUS-guided CNB diagnosis before surgery in our hospital were included in this study. Informed consent was obtained before surgery and biopsy. Approval for the study protocol was given by the hospital ethics committee. Routine blood tests, coagulation tests, and examination for infectious diseases were performed before biopsy. The exclusion criteria were as follows: (I) high position beyond the scope of rectal probe biopsy; (II) incomplete preoperative or postoperative clinicopathologic information; (III) postoperative recurrence; (IV) familial adenomatous polyposis or inflammatory bowel disease; (V) preoperative assessment showing an elevated risk for bleeding and infection after biopsy. Finally, a total of 102 patients were enrolled in this study.
Instruments and methods
Olympus FB-24Q-1 biopsy forceps were used for EFB (Olympus CF-Q260A I, Tokyo, Japan) of rectal lesions. According to tumor condition and patients’ tolerance, 2–3 blocks of tissues were collected, but the lesion en bloc was not resected. An ultrasound instrument (UV800; B-K Medical, Herlev, Denmark) with a rectal three-plane probe (Type 8838, 4–12 MHz) was used. The probe had a real-time double-plane plus end scan plane and a double-plane puncture frame. A disposable CNB needle (18G, 20 mm; Bard, Arizona, USA) was used to collect 2–3 strip-like tissue samples 15 mm in length. A cleaning enema was performed before biopsy, and the interval between the original ENB and the TRUS-guided CNB was about 4–6 days. After surgery, body temperature, blood pressure, white blood cell count, and platelet count were monitored, and postoperative bleeding and infection were evaluated.
Pathologic diagnosis
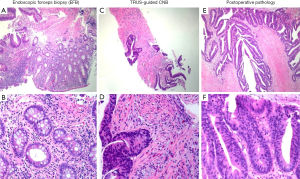
The macroscopic shapes of lesions were classified into three groups: elevated, flat and depressed (17). Rectal tissues were fixed in formalin and embedded in paraffin, and 5-µm consecutive sections were obtained for hematoxylin-eosin staining. According to the WHO diagnostic criteria, HGIN was diagnosed if highly heterotypic glands confined to the lamina propria and the epithelial layer were present, but evidence for ISM was not observed (8). If the tumor had invaded submucosa through the mucosal myometrium, invasive carcinoma was diagnosed. The diagnostic criteria for ISM were as follows: longitudinal stripe-like, mass-like, or focal eosinophilic smooth muscle fibers mixed with heterotypic glands or arranged around the heterotypic glands (Figure 1).
Statistical analysis
Statistical analysis was performed with SPSS, (version 21.0, IBM, Chicago, IL, USA) and Medcalc Statistics (version11.4, MedCalc, Inc., bvba, Belgium). Categorical variables are presented as counts and percentages, whereas continuous variables are expressed as medians with interquartile ranges. Cohen’s kappa value was used as a test of consistency as follows: 0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, excellent (18). Univariate analysis with chi-square test or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables were performed to compare the clinicopathologic characteristics histopathologic discrepancies between EFB and TRUS-guided biopsy. Multivariate analysis with a multiple logistic regression model was performed to identify risk factors for histopathologic discrepancies. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Cut-off values were defined using a receiver operating characteristic (ROC) curve to determine the critical value of risk factors. A value of P<0.05 was considered statistically significant.
Results
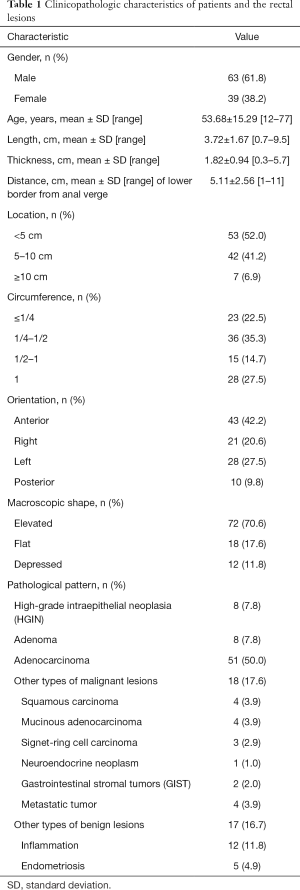
The clinicopathologic characteristics of 102 patients included in this study are summarized in Table 1. Of the 102 patients, 63 were males and 39 were females; the mean age was 53.68±15.29 years (range, 12–77). The main location of the lesions was the lower third of the rectum (52.0%). The main macroscopic shape was elevated 70.6%. Postoperative pathology pattern showed HGIN in 8 patients (7.8%), adenoma in 8 patients (7.8%), adenocarcinoma in 51 patients (50.0%), other malignant lesions in 18 patients (17.6%), and other benign lesions in 17 patients (including inflammation 11.8% and endometriosis 4.9%).
Full table
Comparison pathology type of EFB and TRUS-guided CNB with postoperative pathology
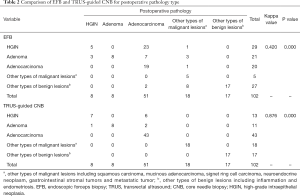
The initial EFB diagnoses of the 102 lesions were HGIN in 29 lesions, adenoma in 21 lesions, adenocarcinoma in 20 lesions, other types of malignant lesions in 5 lesions, and other benign lesions in 27 lesions (Table 2). The TRUS-guided CNB diagnoses of these lesions were HGIN in 13 lesions, adenoma in 11 lesions, adenocarcinoma in 43 lesions, other types of malignant lesions in 18 lesions, and other benign lesions in 17 lesions. The kappa value for consistency with postoperative pathology findings was 0.420 for EFB and 0.876 for TRUS-guided CNB, which showed a significant difference (P<0.01). When the EFB and TRUS-guided CNB diagnoses were compared with those from the postoperative pathology, the histopathologic discrepancy rate was 51.0% (52/102 lesions) and 8.8% (9/102 lesions), respectively (Table 3). The most common discordant diagnosis involved upgrades from HGIN to adenocarcinoma (23/29, 79.3%) and upgrades from adenoma to adenocarcinoma (7/21, 33.3%) and HGIN (3/21, 14.3%) in EFB, whereas in TRUS-guided CNB, the main discrepancies involved upgrades from HGIN to adenocarcinoma (6/13, 46.2%) and upgrades from adenoma to adenocarcinoma (2/11, 18.2%) and HGIN (1/11, 9.1%). It was also challenging to distinguish other types of malignant rectal lesions (such as squamous cell carcinoma, mucinous adenocarcinoma, etc.) in EFB. Instead, false negative results were often obtained (such as inflammation and endometriosis) (8/27, 29.6%) (Table 2). In contrast, the diagnostic accuracy was high in TRUS-guided CNB for differentiating other malignant and benign lesions. No major complications requiring additional care have been observed.
Full table
Full table
Characteristics contributing to histopathologic discrepancies between EFB and TRUS-guided CNB with postoperative pathology
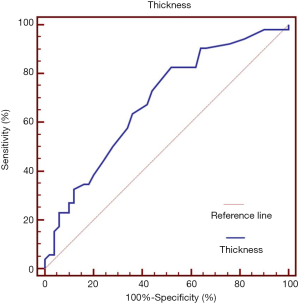
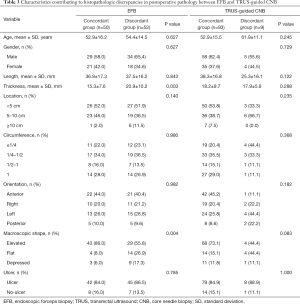
As shown in Table 3, factors such as age, gender, length, location, circumference, orientation, and ulcer did not differ between the concordant and discordant groups in EFB. Compared with the length of lesions, the thickness in EFB had statistical significance. The mean thickness was 20.9±10.2 mm in the discordant groups and 15.3±7.6 mm in the concordant group (P=0.003). The flat and depressed shape occurred more commonly in the discordant (26.9% and 17.3%) than in the concordant (8.0% and 6.0%) group (P=0.004). However, the consistency between TRUS-guided CNB diagnosis and postoperative pathology was not affected by the above factors. Risk factors associated with histopathologic discordant diagnosis using EFB were determined by multivariate analysis (Table 4). Multivariate analysis revealed that thicker thickness [OR 1.080 (95% CI: 1.021–1.142), P=0.007] and flat/depressed shape [OR 0.206 (95% CI: 0.076–0.564), P=0.002] were significantly associated with the discordant group in EFB. The area under the ROC was 0.648 (95% CI: 0.581–0.787, P=0.001) for thickness (Figure 2). For the best cutoff for thickness at 13.5 mm, the sensitivity was 83.7% and the specificity was 52.0%.

Full table
Discussion
Our study is the first to explore the diagnostic value and safety of EFB and TRUS-guided CNB, and the first to determine the risk factors of the histopathologic discrepancies of these two biopsy methods for the purpose of identifying indicators for re-biopsy using TRUS-guided CNB after EFB. A total of 102 patients were included in this retrospective study. No complications were encountered in any of the EFB and TRUS-guided CNB procedures.
In our study, the results showed that the consistencies with postoperative pathology were fair (k=0.402) for EFB, which is consistent with the findings in previous reports (7,12,13). MacDonald et al. (7) reported that 96.6% of patients with preoperative diagnosis of HGIN were diagnosed with invasive cancers by postoperative pathology. In this study, 79.3% with diagnosis of HGIN in EFB were diagnosed with invasive cancers by postoperative pathology. Similarly, underestimation occurs in adenomas which were upgraded to adenocarcinoma (33.3%) and HGIN (14.3%) in EFB. However, TRUS-guided CNB achieved favorable consistency with postoperative pathology (k=0.876). The histopathologic discrepancy rate has been greatly reduced from the initial 51.0% in EFB to 8.8% in TRUS-guided CNB. It was also difficult to distinguish other types of malignant or benign rectal lesions (such as squamous cell carcinoma, mucinous adenocarcinoma, etc., or inflammation and endometriosis) in EFB. Nevertheless, it is not a problem to diagnose these pathological types in TRUS-guided CNB.
These findings might be explained as follows. First, the mean thickness of the colorectal mucosa is about 0.7–0.8 mm (19), and the depth is generally 2–3 mm for EFB. It is theoretically easy to include the muscularis mucosae in the biopsy specimen in normal mucosa. However, in many colorectal cancer patients, biopsy specimens only present dysplastic glands without eosinophilic infiltrate in muscle bundles (muscularis mucosae). Degradation of the muscularis mucosae has also been observed in early colorectal cancer (20-22). In view of the above evidence, it is suggested that the damage of muscularis mucosae by cancer cells contributes to the failure to diagnose ISM. However, TRUS-guided CNB is helpful for the collection of samples containing all 5 layers of the rectal wall (generally 15–22 mm in biopsy depth). Thus, TRUS-guided CNB may achieve elevated accuracy and a lower false negative rate.
Second, the cancerous tissues of adenomas are heterogeneous, and some studies have shown that colorectal cancer is derived from adenoma (11,23-25). Thus, in the malignant transformation of adenoma, some tissues may have characteristics of HGIN or adenocarcinoma. In some cases, typical cancer tissues cannot be sampled via EFB, and only HGIN tissues are collected, which may cause underdiagnosis. Evidence shows that superficial sampling and sampling error are the major causes of failure to confirm ISM (7). Furthermore, the differences in pathologic types and sources provide higher requirements for biopsy tissue and increase the difficulty of pathologic determination, which reduces the diagnostic efficiency of EFB. In contrast, TRUS-guided CNB may be carried out at suspect areas under the guidance of ultrasound, which increases the positive rate of biopsies.
Although EFB is the most common method for clinical biopsy of rectal lesions, it is usually limited by inaccurate diagnosis, which requires re-detect biopsies to assist in treatment decision-making management. Therefore, risk factors for histopathologic discrepancies in EFB should be analyzed to identify suitable patients for re-biopsy using TRUS-guided CNB and avoid overdiagnosis.
In several previous studies, risk factors associated with gastric cancer after surgery for HGIN lesions were discussed. Xu et al. (15) demonstrated the depressed pattern and lesion size ≥2 cm as independent risk factors for upgraded pathology from HGIN to early gastric cancer. Ryu et al. (26) showed that central depression, nodular surface, surface redness, lesion location, large tumor size, and submucosal fibrosis were associated with early gastric cancer or submucosal cancer. This study not only discussed HGIN or submucosal cancer, but also other malignant and benign lesions. In our study, the multivariate analyses and ROC curve indicated that lesions thickness ≥13.5 mm [OR 1.080 (95% CI: 1.021–1.142), P=0.007] and flat/depressed shape [OR 0.206 (95% CI: 0.076–0.564), P=0.002] were significantly associated with histopathologic discrepancies in EFB.
This study had several limitations. First, this was a retrospective study. Thus, a selection bias may have been introduced due to the analyzed cases not being consecutive. Second, patients underwent TRUS-guided CNB nearly 4–6 days later than EFB, which might have produced some bias. In addition, the small sample size was one of the limitations of this study. Further multi-center, large sample size studies are necessary to verify our results.
In conclusion, the current study showed that EFB was of limited clinical value in identifying the preoperative diagnosis of rectal lesions. TRUS-guided CNB is an effective and safe method for preoperative pathologic diagnosis of rectal lesions. When the lesions thickness ≥13.5 mm and flat/depressed shape at initial EFB, TRUS-guided CNB can be considered for application.
Acknowledgments
Funding: This work was supported by the Guangzhou Science and Technology Planning Projects (Health Medical Collaborative Innovation Program of Guangzhou) (grant No. 201803040019).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center (No. C2018-014-01). Informed consent was obtained before surgery and biopsy.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum 2009;52:577-82. [Crossref] [PubMed]
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993;341:457-60. [Crossref] [PubMed]
- Shen C, Yin Y, Chen H, et al. Neuroendocrine tumors of colon and rectum: validation of clinical and prognostic values of the World Health Organization 2010 grading classifications and European Neuroendocrine Tumor Society staging systems. Oncotarget 2017;8:22123-34. [PubMed]
- Benson AB 3rd, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:874-901. [Crossref] [PubMed]
- MacDonald AW, Tayyab M, Arsalani-Zadeh R, et al. Intramucosal carcinoma on biopsy reliably predicts invasive colorectal cancer. Ann Surg Oncol 2009;16:3267-70. [Crossref] [PubMed]
- Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press, 2000.
- West AB, Mitsuhashi T. Cancer or high-grade dysplasia? The present status of the application of the terms in colonic polyps. J Clin Gastroenterol 2005;39:4-6. [PubMed]
- Aust DE, Ruschoff J. Polyps of the colorectum: non-neoplastic and non-hamartomatous. Pathologe 2011;32:297-302. [Crossref] [PubMed]
- Aarons CB, Shanmugan S, Bleier JI. Management of malignant colon polyps: current status and controversies. World J Gastroenterol 2014;20:16178-83. [Crossref] [PubMed]
- Wei XB, Gao XH, Wang H, et al. More advanced or aggressive colorectal cancer is associated with a higher incidence of "high-grade intraepithelial neoplasia" on biopsy-based pathological examination. Tech Coloproctol 2012;16:277-83. [Crossref] [PubMed]
- Tominaga K, Fujinuma S, Endo T, et al. Efficacy of the revised Vienna Classification for diagnosing colorectal epithelial neoplasias. World J Gastroenterol 2009;15:2351-6. [Crossref] [PubMed]
- Wu W, Wu YL, Zhu YB, et al. Endoscopic features predictive of gastric cancer in superficial lesions with biopsy-proven high grade intraepithelial neoplasia. World J Gastroenterol 2009;15:489-95. [Crossref] [PubMed]
- Xu G, Zhang W, Lv Y, et al. Risk factors for under-diagnosis of gastric intraepithelial neoplasia and early gastric carcinoma in endoscopic forceps biopsy in comparison with endoscopic submucosal dissection in Chinese patients. Surg Endosc 2016;30:2716-22. [Crossref] [PubMed]
- Antonini F, Delconte G, Fuccio L, et al. EUS-guided tissue sampling with a 20-gauge core biopsy needle for the characterization of gastrointestinal subepithelial lesions: A multicenter study. Endosc Ultrasound 2019;8:105-10. [Crossref] [PubMed]
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [Crossref] [PubMed]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 2004;36:1094-8. [Crossref] [PubMed]
- Ban S, Shimizu M. Muscularis mucosae in desmoplastic stroma formation of early invasive rectal adenocarcinoma. World J Gastroenterol 2009;15:4976-9. [Crossref] [PubMed]
- Ban S, Kamada K, Mitsuki N, et al. Phenotypic change of muscularis mucosae in early invasive colorectal adenocarcinoma. J Clin Pathol 2000;53:878-81. [Crossref] [PubMed]
- Farina AR, Mackay AR. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers (Basel) 2014;6:240-96. [Crossref] [PubMed]
- Kuramitsu Y, Nakamura K. Proteomic analysis of cancer tissues: shedding light on carcinogenesis and possible biomarkers. Proteomics 2006;6:5650-61. [Crossref] [PubMed]
- Shussman N, Wexner SD. Colorectal polyps and polyposis syndromes. Gastroenterol Rep (Oxf) 2014;2:1-15. [Crossref] [PubMed]
- Bujanda L, Cosme A, Gil I, et al. Malignant colorectal polyps. World J Gastroenterol 2010;16:3103-11. [Crossref] [PubMed]
- Ryu DG, Choi CW, Kang DH, et al. Clinical outcomes of endoscopic submucosa dissection for high-grade dysplasia from endoscopic forceps biopsy. Gastric Cancer 2017;20:671-8. [Crossref] [PubMed]