
The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype?
Introduction
Red Blood Cell distribution width (RDW) is a simple measure of the broadness of erythrocyte size distribution, conventionally called anisocytosis (1). This measure is easily, inexpensively and rapidly calculated as ratio of standard deviation (SD) of red blood cell (RBC) volume and mean corpuscular volume (MCV) [i.e., (RDW-SD)/(MCV)×100], with final result expressed as percentage (2). The modern hematologic analyzers measure RDW by either with impedance or optical techniques, and this different approach contributes to make inter-instrument and inter-laboratory comparison rather challenging (3). Commercial hematological analyzers also differ in size limits and relative height of RBC histogram used for calculation, thus making harmonization of measurements a virtually unreachable target.
Despite these technical issues that would contribute to challenge harmonization, RDW is automatically calculated in almost every patient in whom a complete blood count (CBC) is requested. Although the reference range typically spans between 12–15%, the so-called normal values are largely dependent on instrumentation and population, so that the single laboratory shall establish its own local confidence intervals (2). The first and most clinically significant use of RDW is for evaluating different types of anaemias. Several forms of anaemia may manifest with high RDW, and this clear-cut finding would then be very helpful for the differential diagnosis. For example, RDW is useful in the differential diagnosis between iron deficiency anaemia and heterozygous thalassemia, whereby RDW is higher in the former condition and almost normal in the latter. Other forms of anaemia with larger RDW encompass haemolytic anaemias, hereditary spherocytosis, vitamin B12 and/or folate deficiency, anaemia related to myelodysplastic syndrome and so forth. RDW can also increase in other conditions such as blood transfusion, chronic liver disease (4), autoimmune disorders (5) and cancer (6).
Factors affecting the RDW
Beside anaemia, many factors have been associated with RDW variation such as age, sex, genetic factors, renal function and dyslipidaemia (4). We will hence summarize here some published evidence.
Regarding ageing, Lippi and colleagues showed that RDW values tend to increase in parallel with the age of subjects, becoming consistently high after the age of 60 years (7). In a subsequent study, Hoffmann confirmed these findings (8) using two different haematological analyzers, finally reporting that MCV is also age-dependent. Since MCV is a parameter used for calculating the RDW, it was hence concluded that the association between RDW and age might be at least partially mediated by MCV.
Gender can also influence RDW, though the available evidence is not always straightforward or concordant. Two studies (7,9) concluded that women have on average a lower RDW compare to men, whilst higher values have also been observed in Black populations compared to Whites (9).
The most comprehensive information on metabolic factors potentially associated with RDW emerged from a large study, involving 3,529 consecutive patients undergoing coronary angiography as part of the Tel Aviv Prospective Angiographic Survey (TAPAS) (10). In this population the various components of the metabolic syndrome, either taken as dichotomized or continuous variables, were found to be significantly associated with increased RDW values. Similar associations were seen with the metabolic syndrome defined by the Adult Treatment Panel III (ATPIII) criteria, after adjusting for age, gender, anaemia, white blood cell count, MCV and ischaemic heart disease (10). In the same study, inflammatory biomarkers such as C-reactive protein (CRP), white blood cells and fibrinogen were also found to be significantly associated with higher RDW. Finally, the RDW value independently predicted mortality and CV co-morbidities such as hypertension, diabetes mellitus (DM), peripheral artery disease and ischaemic cardiac disease (10).
A recent genome wide association study (GWAS) based on over 100,000 UK Biobank human volunteers concluded that ~30% of RDW variance could be explained by 457,643 directly genotyped single nucleotide polymorphisms (SNPs; MAF >0.1%), 30,988 of which, mapped to 141 loci, were highly significantly (P<5×10–8) associated with RDW (11). After conditional analysis, three exonic missense variants belonging to the protein function genes TRIM58, PLD1 and PNPLA3 were shown to have in silico “probably damaging” effect (11). Interestingly, direct comparison with the catalogue of published GWAS, allowed identifying individual RDW variants associated with iron metabolism and other RBC cell measures, along with body mass index (BMI), lipids, hemoglobin A1c (HbA1c), metabolic syndrome, autoimmune and inflammatory diseases (11). Even more interestingly, Pilling and colleagues, used several genetic risk score previously associated with different traits and disease, and found that most of these, especially those related to cholesterol [both low-density lipoprotein (LDL) and high-density lipoprotein (HDL)], triglycerides, systolic blood pressure (SBP), BMI, type 1 diabetes and some inflammatory diseases were significantly associated with RDW (11).
In another study involving 40 patients with dyslipidemia, HDL, triglycerides small and dense LDL subfraction and apolipoprotein B-containing lipoproteins were found to be significantly associated with RDW values. Atorvastatin treatment was effective to concomitantly modify some of these variables, and the net effect was mirrored by a decline of RDW values, thus suggesting that this parameter may also be used for monitoring treatment with lipid lowering drugs (12).
In another large retrospective study, 2,688 subjects without DM at baseline underwent routine medical examination and were then followed up for 4 years. After adjustment for HbA1c and high sensitivity CRP, the multivariate relative risk of developing DM was significantly enhanced in the highest quartile of RDW compared to the lowest (13).
In another cross-sectional study, including healthy Chinese people who participated to a health check-up, blood pressure (BP) was systemically measured in over 300,000 patients. According to cut off values of ≥140 mmHg or/and over ≥90 mmHg for BP, or medication for controlling BP, the authors found that hypertension, SBP and diastolic BP displayed an inverted U-shaped relationship with RDW values, exhibiting also different peaks between genders (14). In another cross-sectional study an association between RDW and circadian patterns of BP was identified. Specifically, 708 patients with essential hypertension were divided in three groups, dipper (mean decrease of SBP during sleeping of 10–20%), non-dipper (less than 10% of nocturnal SBP fall) and reverse dipper (rising of SBP during sleeping) according to result of ambulatory BP monitoring (15). After adjustment for covariates, RDW was found to be significantly associated with circadian BP pattern, in particular with reverse dipper patterns. RDW was also different in non-dipper pattern compared with dipper pattern, but no differences in RDW could finally be noted between reverse dipper and non-dipper patterns (15). This finding reinforces the hypothesis of the existence of an association between BP and RDW.
Loprinzi et al. also explored the possible association of RDW and physical activity in 4,535 healthy subjects from the National Health and Nutrition Examination Surveys (NHANES), observing that these two parameters were inversely related after adjusting for many covariates (16). This would actually mean that physical inactivity, a major risk factor for CV diseases, might also be accompanied by increased RDW values.
Regarding kidney function as a potential determinant of RDW, a study based on 195 patients with clinical suspected heart failure (HF) found that subjects belonging to the highest tertile of RDW has a greater burden of impaired renal function, defined as glomerular filtration rate (GFR) <55 mL/min/1.73 m2 (17). In a more recent study involving 513 patients with essential hypertension, Li and colleagues reported a statistically significant association between RDW and SBP or albumin-to-creatinine ratio after adjustment for potential confounders such as age, sex and hemoglobin (18).
According to the above-mentioned studies, it would seem reasonable that RDW can be influenced by several demographic and biological factor such as age, physical activity, hypertension, metabolic syndrome, renal and liver function, inflammation and, last but not least, genetic factors.
RDW as a diagnostic tool
Since evidence has been provided that RDW is a risk factor for CV diseases, its diagnostic performance has been widely evaluated. In 2011 Cemin et al. carried out a study on 1,971 chest pain patients [133 with a final diagnosis of acute myocardial infarction (AMI)], reporting that RDW was capable to predict AMI diagnosis with 61% accuracy in women [area under the curve (AUC), 0.610] (19). A significant but lower performance was also observed in men (AUC, 0.591). In a subsequent study performed including 2,304 adult patients consecutively admitted over a 1-year period to the emergency department for chest pain (20), RDW predicted AMI with 71% accuracy (AUC, 0.705). Although the performance of RDW was obviously found to be lower than that of cardiac troponins, the authors highlighted that in the 25 AMI patients with negative cardiac troponin values on admission, 21 of them (84%) had RDW values above the local diagnostic cut-off, thus suggesting that the combination of RDW and cardiac troponin may improve the diagnostic accuracy.
Only one study explored the diagnostic performance of RDW in stroke patients, revealing that this parameter exhibited 89% diagnostic accuracy (AUC, 0.89; sensitivity and specificity of 0.74 and 0.88 respectively) for discriminating stroke subtypes in young adults (21). Unfortunately, the design of this study was retrospective and cross-sectional, whilst the sample size was relatively small, so that further prospective studies may be warranted before definitive conclusions could be drawn on this matter.
RDW as risk and prognostic factor
CAD and mortality
Both the Malmö Diet and Cancer (MDC) and the Trömso study are large, urban-based prospective cohort studies based in northern Europe including a large sample of initially healthy people (more than 25,000 for both) aged between 40–70 years, who are prospectively followed up for many different endpoints. In the Trömso study, RDW was found to be independently associated with future AMI after adjusting the analysis for several parameters including CV risk factors, displaying a hazard ratio (HR) of 1.34 (95% CI, 1.11–1.60; fourth quartile compared to the first quartile of RDW) (22). In the MDC study RDW was measured in more than 26,000 subjects without pre-existing AMI or stroke. After a mean follow-up of 14 years, people in the highest quartile of RDW at baseline had a 1.8-fold enhanced risk of incident fatal acute coronary events after adjusting for several covariates. No association was instead observed between RDW and incidence of non-fatal coronary events (23). In the UK Biobank study, including 240,477 healthy volunteers aged 40–70 years and followed up for an average period of 9 years, the incidence of CAD and all-cause mortality began to increase with RDW values >13% and was the highest (i.e., nearly 3-fold higher) in participants with RDW value >15% (24). In the NHANES study, the 10-year coronary heart disease (CHD) risk-adjusted HR was higher in subjects with elevated RDW values after adjustment for CV risk factors and factors related to anaemia and vitamin B complex deficiency (25). In another survey of the NHANES, all-cause mortality was substantially higher in subjects in the highest RDW quartile, especially in men (9).
In other studies the clinical value of RDW was also explored in subjects with pre-existing CAD. Ren et al. studied 1,442 Chinese patients with stable angina pectoris, and found that RDW was significantly associated with both mortality and acute coronary syndrome (ACS) after adjusting for several factors including values of left ventricular ejection fraction (LVEF) and brain natriuretic peptide (BNP) (26). In another very large study including patients with ST elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI), an increased RDW value % was associated with long-term CV mortality both in anaemic and non-anaemic patients, independently from CV risk factors (27). In another case-control study 146 patients admitted with ACS and stent thrombosis after a previous successful PCI were compared with 175 patients who had similar procedural characteristics but without stent thrombosis. RDW was found to be an independent predictor of stent thrombosis in multivariate logistic regression analysis (28). In another observational study in 312 STEMI patients undergoing thrombolytic therapy, baseline RDW was found to be an independent predictor of resolution of ST segment elevation and in hospital occurrence of major adverse cardiac events (MACE) (29).
In a post-hoc analysis of a randomized controlled trial (RCT) where CAD patients were randomized to receive pravastatin 40 mg daily or placebo (Cholesterol and Recurrent Events study), RDW was associated with coronary and all-cause mortality (30) independently from CV risk factors, GFR and anaemia.
In another study Horne and colleagues (31) highlighted a possible limitation of RDW. Since this marker was found to be dependent on MCV (i.e., MCV is placed at the denominator in the RDW calculations), and MCV was independently associated with mortality in several studies, it was finally concluded that adverse outcomes may be better predicted by expressing RDW as standard deviation of RBC volumes (i.e., RDW-SD) rather than using its conventional expression as coefficient of variation (i.e., RDW-CV). This conclusion was based on results of their study, which showed that RDW-SD (distributed in quintiles) was more strongly associated with all-cause mortality than RDW-CV (also distributed in quintiles) after adjusting for several covariates (HR, 8.4 versus 4.8; P<0.001).
In 2014, Su and colleagues summarized in a meta-analysis the possible association between RDW and mortality or CV events, including 9 primary studies and totalling 55,905 patients (32). The overall HR was 2.20 (95% CI, 1.4–3.4) though the funnel plot showed suggestive bottom asymmetry, reflecting publication bias for smaller studies. Four years later, another meta-analysis using almost identical inclusion and exclusion criteria but focusing in patients with ACS, found that a low RDW value is associated with decreased mortality (RR, 0.35; 95% CI, 0.30–0.40) (33). In this second meta-analysis the funnel plot appeared almost symmetrical, thus compatible with no publication bias.
Stroke
A number of studies also explored the potential clinical usefulness of RDW for prognosticating patients with ischaemic cerebral disease. In a case-control study including 124 consecutive patients with a first-ever ischemic stroke and 124 matched controls, RDW displayed a graded, increasing association with ischemic stroke across quartiles, exceeding 4-fold when people in the highest RDW quartile were compared with those in the lowest quartile (34).
In a large population-based cohort study 25,992 participants were studied at baseline and then followed up for a median period of 16 years (35). After adjustment for smoking habit, hemoglobin level, leukocyte and platelet counts, hypertension, cholesterol, triglycerides and self-reported DM, people in the highest RDW quintile had a 37% enhanced risk of incident stroke compared to those in the lowest quintile. Interestingly, the relationship between RDW and stroke was found to be linear across RDW quintiles, with 13% higher risk for each 1% increment (35). In the urban-based MDC study, the relation between baseline RDW and risk of incident stroke was confirmed (31% increased risk between highest and lowest quartiles), whilst a relationship between RDW and intima-media thickness could also be identified (36). A study conducted in Israel and based on 41,140 patients with atrial fibrillation followed up for 38,024 person-years (37) conclude that the cumulative risk of stroke gradually increased across quartile of RDW, independently from the presence of anaemia and after adjustment for variables of the CHADS2 score.
Turcato et al. studied 316 ischemic stoke patients undergoing thrombolytic therapy, and observed an over 6-fold higher mortality and 1.8-fold lower stroke recovery in patients with RDW values above the local cut-off (38). In another retrospective study of 847 patients with first-ever acute cerebral infarction, increased RDW was found to be associated with poor functional outcome (+1.2-fold per each 1% increment), as well as with all-cause mortality (+1.4-fold per each 1% increment) during follow-up (39). Besides ischemic stroke, increased RDW has also been associated with worse prognosis in patients with subarachnoid haemorrhage (SAH). In particular, Huang investigated the prognostic value of RDW in 274 SAH patients and found that that 1-year mortality increased by 20% per each 1% increment of RDW (40).
Peripheral vascular disease
As regards peripheral arterial disease (PAD), Demirtas et al. prospectively followed up 82 patients with this condition, and found that RDW values gradually grow in parallel with increasing Fontaine’s stages (P<0.05 for trend) (41). In 6,950 participants of the National Health and Nutrition Examination Survey (NAHNES 1999–2004) RDW values were also found to be directly associated with the risk of PAD and inversely correlated with ankle to brachial index (ABI), which mirrors lower BP at lower extremities (42). In participants with ABI <0.9, including RDW to the estimated Framingham risk score could enhance the diagnostic performance for predicting PAD (e.g., the AUC increased from 0.66 to 0.73). In 3,039 consecutive outpatients with PAD identified by non-invasive lower-extremity arterial testing at the Mayo Clinic, RDW was also found to be a significant predictor of mortality (i.e., 66% higher risk in the highest compared to the lowest RDW quartile) (43).
Atrial fibrillation
The first report on the possible relationship between RDW and atrial fibrillation (AF) was published by Eryd et al. in 2014 (44). A total number of 27,124 subjects free of AF at baseline were recruited and followed up for 13.6 years. The population was divided into quartiles of baseline RDW values and those in the highest quartile had a 1.33 higher risk of incident AF than those in the lowest quartile. These findings were then confirmed in another study on 240,477 healthy UK Biobank volunteers (77% higher risk of incident AF in patients with values >15%) (24) and by a meta-analysis of observational studies evaluating hematologic parameters in patients with new-onset AF and recurrent AF (weight mean difference of 0.28% in subjects developing incident AF) (45).
In patients with AF, increased RDW was also found to be associated with worse outcomes. In a prospective study including 300 AF patients followed-up for a mean period of 3.2 years (46) RDW was found to be independently associated with MACE (1.01-fold increase per each 1% RDW increment) and all-cause mortality (1.02-fold increase per each 1% RDW increment). In another large retrospective study, including 41,140 adult AF patients, stroke incidence rate gradually increased across RDW quartiles (37). The association between RDW and stroke was not dependent upon the presence of anaemia. Recent evidence has also been provided that RDW may improve the predictive accuracy of CHADS2 score in stroke patients. Cha and colleagues explored the association between RDW and thromboembolic events (including ischemic stroke and systemic embolism) in 5,082 consecutive patients with non-valvular AF (47), and found that increased RDW was independently associated with an enhanced risk (1.6-fold compared to normal values) of thromboembolic events after adjusting for age, hypertension, vascular disease, and previous stroke history.
Heart failure
In 2007, a post-hoc evaluation of the CHARM trial which assessed the effect of candesartan and angiotensin II blockers in 2,679 symptomatic chronic HF patients, showed that subjects in the highest quintile of RDW had a much higher risk of CV death or hospitalization for HF than those in the lowest quintile (HR, 1.64). These findings were then replicated, at least for the mortality endpoint, in 2,140 HF patients from the Duke Databank (48), whereby each 1 SD increment of RDW was associated with a 1.29 higher risk of CV and 1.17 higher risk of death or hospitalization for HF. In another study including 6,159 consecutive ambulatory patients with chronic HF, an RDW >16% at baseline was associated with an increased risk of death during the follow-up compared with RDW <16% (49). Notably, each 1% increment of RDW at baseline was associated with 1.17-fold higher risk of all-cause mortality. Interestingly, not only the baseline RDW was predictive of adverse outcome, but also an increasing RDW during follow-up was found to be significant predictor of all-cause mortality (1.08-fold higher risk for each 1% increase in RDW during follow up) (49). Xanthopoulos et al. measured RDW in 218 patients admitted for acute decompensated HF, and reported that baseline RDW was associated with higher risk of HF events in both patients with and without DM (by 1.35- and 1.14-fold, respectively), whilst it longitudinal changes were also predictive of HF episodes (50). Uemura and colleagues studied 229 patients with acute decompensated HF, and observed that those with higher RDW at baseline (HR, 1.05) or positive changes during follow-up (HR, 1.25) had a worst prognosis, especially for cardiac-based mortality (51). In another study based on 278 patients with acute decompensated HF with preserved ejection fraction (HFpEF), baseline RDW >14.7% predicted higher all-cause (HR, 1.15) and non-cardiac mortality (HR, 1.18) after adjustment for CV risk factors, inflammation, anaemia and nutritional deficiency (52). Even more importantly, RDW also improved the net reclassification index (NRI) and the integrated discrimination index (IDI), thus supporting its implementation with traditional prognostic scores (52).
Different systematic reviews with meta-analyses attempted to summarize the information on RDW and subsequent CV risk, especially HF. In particular the meta-analysis by Huang et al. (53) including 9 studies and totaling 18,288 HF patients, concluded that each 1% increase in RDW was associated with a 1.10-fold higher risk (95% CI, 1.06–1.14) of all-cause mortality. The meta-analysis of Shao and colleagues (54), which included 17 studied and 41,311 patients concluded that each 1% increase in RDW was associated with a 1.09-fold increase risk of HF hospitalization and 1.19-fold increased risk of combined adverse events. A more recent meta-analysis has been published by Hou et al., including 28 studies and totalling 102,689 patients (55). Overall, each 1% increase of RDW was associated with 1.12-fold higher risk of all-cause mortality and a similarly 1.12-fold higher risk of MACE.
In subjects free of HF, increased RDW is also associated with higher HF risk. In over 17,000 healthy participants from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort, the composite endpoint of HF free survival (hospitalization or death due to HF) was significantly different across quartiles of RDW (56) after adjustment for CV risk factors, anaemia and even ferritin level. Almost identical outcome was reached in the MDC study, where the first hospitalization due to HF increased in parallel with RDW quartiles (57).
To summarize the main technical and biological aspects of RDW in cardiovascular risk assessment, some pros and cons can be identified, as summarized in Table 1.
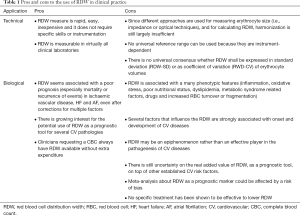
Full table
Conclusions
Taken together, the current evidences could be summarized as follows. RDW as a diagnostic tool (i.e., in the evaluation of chest pain) is probably non-specific, though it could provide some useful additional information beyond consolidate biomarkers such as cardiac troponins. Nonetheless, RDW is may be instead a clinically useful parameter for predicting the future development and the prognosis of many CV diseases, such as stroke, AF and HF. Evidence of an increase RDW should thus persuade clinicians to (I) investigate possible causes of anaemia (even when anaemia is not yet present), especially due to malnutrition/malabsorption (iron, folate, cianocobalamin); (II) consider that patients with high RDW are at enhanced risk of clinical worsening and/or death, and shall hence be more accurately investigated and more aggressively managed for their incident diseases (i.e., targeting specific risk factors that can have an adverse impact on prognosis such as impaired kidney function, low physical activity, metabolic syndrome, inflammation etc.); (III) detect which other specific factors may be responsible for an increased RDW value and, whenever possible, promptly establishing corrective measure to bring back RDW within normal reference limits.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med 2014;52:1247-9. [Crossref] [PubMed]
- Lippi G, Pavesi F, Bardi M, et al. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem 2014;47:1100-3. [Crossref] [PubMed]
- Lippi G, Turcato G, Cervellin G, et al. Red blood cell distribution width in heart failure: A narrative review. World J Cardiol 2018;10:6-14. [Crossref] [PubMed]
- Hu ZD. Red blood cell distribution width: a promising index for estimating activity of autoimmune disease. J Lab Precis Med 2016;1:4. [Crossref]
- Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med 2016;4:399. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med 2014;52:e197-9. [PubMed]
- Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med 2015;53:2015-9. [Crossref] [PubMed]
- Zalawadiya SK, Veeranna V, Panaich SS, et al. Gender and ethnic differences in red cell distribution width and its association with mortality among low risk healthy United state adults. Am J Cardiol 2012;109:1664-70. [Crossref] [PubMed]
- Laufer Perl M, Havakuk O, Finkelstein A, et al. High red blood cell distribution width is associated with the metabolic syndrome. Clin Hemorheol Microcirc 2015;63:35-43. [Crossref] [PubMed]
- Pilling LC, Atkins JL, Duff MO, et al. Red blood cell distribution width: Genetic evidence for aging pathways in 116,666 volunteers. PLoS One 2017;12:e0185083. [Crossref] [PubMed]
- Kucera M, Balaz D, Kruzliak P, et al. The effects of atorvastatin treatment on the mean platelet volume and red cell distribution width in patients with dyslipoproteinemia and comparison with plasma atherogenicity indicators--A pilot study. Clin Biochem 2015;48:557-61. [Crossref] [PubMed]
- Gang L, Lifang W. Association of the elevated red blood cell distribution width with the risk of developing diabetes mellitus. Intern Med 2016;55:1959-65. [Crossref] [PubMed]
- Jiang M, Zha X, Wu Z, et al. Inverted U-shaped curve relationship between red blood cell distribution width and hypertension in a large health checkup population in China. J Am Soc Hypertens 2018;12:327-34. [Crossref] [PubMed]
- Su D, Guo Q, Gao Y, et al. The relationship between red blood cell distribution width and blood pressure abnormal dipping in patients with essential hypertension: a cross-sectional study. BMJ open 2016;6:e010456. [Crossref] [PubMed]
- Loprinzi PD, Hall ME. Physical activity and dietary behavior with red blood cell distribution width. Physiol Behav 2015;149:35-8. [Crossref] [PubMed]
- Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659-66. [Crossref] [PubMed]
- Li ZZ, Chen L, Yuan H, et al. Relationship between red blood cell distribution width and early-stage renal function damage in patients with essential hypertension. J Hypertens 2014;32:2450-5. [Crossref] [PubMed]
- Cemin R, Donazzan L, Lippi G, et al. Blood cells characteristics as determinants of acute myocardial infarction. Clin Chem Lab Med 2011;49:1231-6. [Crossref] [PubMed]
- Lippi G, Filippozzi L, Montagnana M, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med 2009;47:353-7. [Crossref] [PubMed]
- Demir R, Saritemur M, Atis O, et al. Can we distinguish stroke and stroke mimics via red cell distribution width in young patients? Arch Med Sci 2015;11:958-63. [PubMed]
- Skjelbakken T, Lappegård J, Ellingsen TS, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the tromsø study. J Am Heart Assoc 2014;3:e001109. [Crossref] [PubMed]
- Borné Y, Smith JG, Melander O, et al. Red cell distribution width in relation to incidence of coronary events and case fatality rates: a population-based cohort study. Heart 2014;100:1119-24. [Crossref] [PubMed]
- Pilling LC, Atkins JL, Kuchel GA, et al. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One 2018;13:e0203504. [Crossref] [PubMed]
- Zalawadiya SK, Veeranna V, Niraj A, et al. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol 2010;106:988-93. [Crossref] [PubMed]
- Ren H, Hua Q, Quan M, et al. Relationship between the red cell distribution width and the one-year outcomes in Chinese patients with stable angina pectoris. Intern Med 2013;52:1769-74. [Crossref] [PubMed]
- Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011;22:138-44. [Crossref] [PubMed]
- Tunçez A, Çetin MS, Çetin EH, et al. Association between RDW and stent thrombosis in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Medicine (Baltimore) 2017;96:e5986. [Crossref] [PubMed]
- Ghaffari S, Pourafkari L, Sepehrvand N, et al. Red cell distribution width is a predictor of ST resolution and clinical outcome following thrombolysis in acute ST elevation myocardial infarction. Thromb Res 2016;140:1-6. [Crossref] [PubMed]
- Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008;117:163-8. [Crossref] [PubMed]
- Horne BD, Muhlestein JB, Bennett ST, et al. Association of the dispersion in red blood cell volume with mortality. Eur J Clin Invest 2015;45:541-9. [Crossref] [PubMed]
- Su C, Liao LZ, Song Y, et al. The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: a systematic review and meta-analysis. J Thorac Dis 2014;6:1429-40. [PubMed]
- Abrahan LL 4th, Ramos JDA, Cunanan EL, et al. Red cell distribution width and mortality in patients with acute coronary syndrome: a meta-analysis on prognosis. Cardiol Res 2018;9:144-52. [Crossref] [PubMed]
- Ramirez-Moreno JM, Gonzalez-Gomez M, Ollero-Ortiz A, et al. Relation between red blood cell distribution width and ischemic stroke: a case-control study. Int J Stroke 2013;8:E36. [Crossref] [PubMed]
- Lappegård J, Ellingsen TS, Skjelbakken T, et al. Red cell distribution width is associated with future risk of incident stroke. The tromsø study. Thromb Haemost 2016;115:126-34. [Crossref] [PubMed]
- Söderholm M, Borné Y, Hedblad B, et al. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: a population-based cohort study. Plos One 2015;10:e0124957. [Crossref] [PubMed]
- Saliba W, Barnett-Griness O, Elias M, et al. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med 2015;128:192.e11-8. [Crossref] [PubMed]
- Turcato G, Cappellari M, Follador L, et al. Red blood cell distribution width is an independent predictor of outcome in patients undergoing thrombolysis for ischemic stroke. Semin Thromb Hemost 2017;43:30-5. [Crossref] [PubMed]
- Kim J, Kim YD, Song TJ, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost 2012;108:349-56. [Crossref] [PubMed]
- Huang YL, Han ZJ, Hu ZD. Red blood cell distribution width and neutrophil to lymphocyte ratio are associated with outcomes of adult subarachnoid haemorrhage patients admitted to intensive care unit. Ann Clin Biochem 2017;54:696-701. [PubMed]
- Demirtas S, Karahan O, Yazici S, et al. The relationship between complete blood count parameters and Fontaine’s Stages in patients with peripheral arterial disease. Vascular 2014;22:427-31. [Crossref] [PubMed]
- Zalawadiya SK, Veeranna V, Panaich SS, et al. Red cell distribution width and risk of peripheral artery disease: analysis of national health and nutrition examination survey 1999-2004. Vasc Med 2012;17:155-63. [Crossref] [PubMed]
- Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol 2011;107:1241-5. [Crossref] [PubMed]
- Eryd SA, Borné Y, Melander O, et al. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med 2014;275:84-92. [Crossref] [PubMed]
- Weymann A, Ali-Hasan-Al-Saegh S, Sabashnikov A, et al. Prediction of new-onset and recurrent atrial fibrillation by complete blood count tests: a comprehensive systematic review with meta-analysis. Med Sci Monit Basic Res 2017;23:179-222. [Crossref] [PubMed]
- Wan H, Yang Y, Zhu J, et al. The relationship between elevated red cell distribution width and long-term outcomes among patients with atrial fibrillation. Clin Biochem 2015;48:762-7. [Crossref] [PubMed]
- Cha MJ, Lee HS, Kim HM, et al. Association between red cell distribution width and thromboembolic events in patients with atrial fibrillation. Eur J Intern Med 2017;46:41-6. [Crossref] [PubMed]
- Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007;50:40-7. [Crossref] [PubMed]
- Cauthen CA, Tong W, Jain A, et al. Progressive rise in red cell distribution width is associated with disease progression in ambulatory patients with chronic heart failure. J Card Fail 2012;18:146-52. [Crossref] [PubMed]
- Xanthopoulos A, Giamouzis G, Melidonis A, et al. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc Diabetol 2017;16:81. [Crossref] [PubMed]
- Uemura Y, Shibata R, Takemoto K, et al. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. J Cardiol 2016;67:268-73. [Crossref] [PubMed]
- Imai R, Uemura Y, Okumura T, et al. Impact of red blood cell distribution width on non-cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J Cardiol 2017;70:591-7. [Crossref] [PubMed]
- Huang YL, Hu ZD, Liu SJ, et al. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. Plos One 2014;9:e104861. [Crossref] [PubMed]
- Shao Q, Li L, Li G, et al. Prognostic value of red blood cell distribution width in heart failure patients: a meta-analysis. Int J Cardiol 2015;179:495-9. [Crossref] [PubMed]
- Hou H, Sun T, Li C, et al. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci Rep 2017;7:43420. [Crossref] [PubMed]
- Emans ME, Gaillard CA, Pfister R, et al. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int J Cardiol 2013;168:3550-5. [Crossref] [PubMed]
- Borné Y, Smith JG, Melander O, et al. Red cell distribution width and risk for first hospitalization due to heart failure: a population-based cohort study. Eur J Heart Fail 2011;13:1355-61. [Crossref] [PubMed]


