A right atrium metastasis of breast cancer after long-term endocrine therapy
Introduction
Cardiac tumors are encountered infrequently in oncological practice. Secondary or metastatic heart tumors are more common than primary tumors (1). The most common primary metastases to the heart are those of the lung, esophagus, melanoma, lymphoma, and breast (2). However, most cardiac transplantations occur within the pericardium, and intra-cardiac metastases are quite unusual. Cardiac metastases may be encountered more frequently during tumor therapy as diagnostic devices become more widely available, and patients live longer due to improved treatment regimens. Because symptoms of metastatic disease are common, signs of cardiac involvement are often overlooked. We report a case of advanced breast cancer with cardiac intracavitary metastasis.
Case presentation
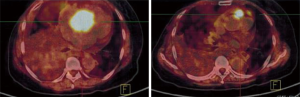
The patient has a history of breast cancer for almost 24 years. A modified radical mastectomy was taken in 1992 when she was 42 years old, and the immunohistochemistry showed ER (+++), PR (-), and C-erbB2 (-) of the right breast. After surgery, she received adjuvant chemotherapy with epirubicin and cyclophosphamide for 2 cycles, followed by treatment with tamoxifen for 7 years. After being diagnosed with the metastasis to lung and mediastinal lymph nodes in 2008, she successively received chemotherapy with docetaxel and capecitabine for 6 cycles and endocrine therapy with letrozole for 16 months, followed by treatment with fulvestrant after progression (Figure 1). This patient had been continuously responsive to fulvestrant for 5 years prior until the atrial lesions were found by echocardiography when she presented with the symptoms. Echocardiography shows two masses in the right atrium and right atrioventricular ring. In addition, it was found that the hypermetabolism image was clear in the right atrium in PET-CT (Figure 2), which influenced the heart ejection function. After evaluation and discussion with the surgical department, a neoplasm resection in the atrium was performed with the aim to alleviate the heart symptom.
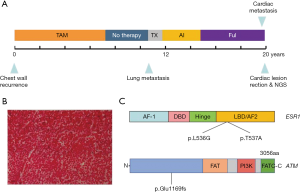
Four tumors were found located respectively in the cardiophrenic surface, the inner wall of the right atrium, and atrial septum, the largest of which (nearly 5 cm × 6 cm) was in the atrioventricular septum near the tricuspid valve. Due to the particularity of the position, the tumor could not be completely removed, and partial resection was performed. After pathological examination, the tumor was confirmed to be breast cancer metastasis with neuroendocrine characteristics (Figure 1B). The immunohistochemistry showed ER (+, 60%), PR (-), C-erbB2 (-), and Ki-67 (20%).
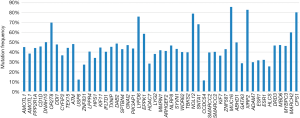
The patient was too weak to receive chemotherapy, and endocrine therapy had exhausted all available drugs at that time, making targeted drugs a possible treatment option. After communicating with the patient and her family members, next-generation sequencing (NGS) of the whole exon from the metastasis was performed to explore the gene expression and look for potential targeted therapeutic opportunities. NGS revealed no aberration in common tumor genes, such as ERBB2, PI3KCA, EGFR, RAS, BRAF, and BRCA genes; however, the other 48 genes were identified with mutations (Figure 3), including ATM frameshift variant and ESR1 missense variant (Figure 1C). Based on the NGS finds, there were no targeted therapies approved by the FDA or CFDA, and the patients received mild therapy and treatment to relieve symptoms.
Discussion
Cardiac metastases have been reported to have an incidence of 1.6% to 20%, but endocardial and intracavitary metastases are more rare, accounting for 3% to 5% of cardiac metastasis cases (3). It had been previously thought that the heart was usually not receptive to malignant tumor cells for the kneading action and the metabolic peculiarity of the heart muscle and rapid blood flow. But, with the development of radiography, such as echocardiography and computed tomography, the primary or secondary cardiac tumors have been detected more frequently, but still with a low incidence. The cardiac metastasis of malignant tumors has received increasing attention in recent years, but there is still no expert consensus or guidelines for treatment and examinations.
Clinically, secondary heart tumors usually remain silent. There are no physical or laboratory examinations that specifically detect cardiac metastasis tumors, and pathology has remained the gold diagnostic standard (1). The method of choice to detect cardiac metastases and their complications is echocardiography. Echocardiography can identify involvement of the valves and their competency, ventricular function, irregular pericardial thickening and intracavity masses interfering with blood flow (4,5). In addition, MRI and computed tomography are useful diagnostic tools to help detect metastases and determine the resectability of tumors (6,7). Computed tomography has a high tissue resolution that can help with tissue characteristics. Cardiac MRI helps to assess the extent of the tumor and the involvement of blood vessels and pericardium. However, MRI cannot distinguish between benign and malignant tumors. Rarely seen studies using PET-CT to detect cardiac metastases, one study showed that PET-CT shows characteristic echocardiographic features (8). In this study, PET-CT, like echocardiography, clearly shows the location of the mass and blood flow signals, further suggesting the potential value of PET-CT in the diagnosis of cardiac metastases.
As the consensus for general treatment is not strong, the treatment may be individual. Cardiac treatment is mostly confined to palliative measures. Surgery is the cornerstone of therapy. However, a multi-treatment approach, including chemotherapy, radiation might provide a better palliative and curative result (2). Surgical resection is indicated in cases of solitary intracavitary heart metastases, which lead to the obliteration of cardiac chambers or valve obstruction (9); however, the outcomes are unclear. A few studies have shown that radiotherapy is a potentially effective local treatment for inoperable patients and that cardiac lesions may respond well to radiotherapy (10). In this case, after full discussion and consideration, surgical resection of the lesion as a palliative treatment was decided. Surgical resection of the lesion gave us the opportunity to be clear about the pathological and biological characteristics. Pathological results showed that the heart disease was derived from breast cancer, but due to poor physical condition after surgery, radiotherapy for residual lesions and systemic chemotherapy could not be tolerated by the patient.
The next-generation sequence of the cardiac lesion was taken in order to find the potential therapeutic approaches, and the possible mechanism of the rare metastasis location. Fifty-one somatic mutations in 48 genes were detected at the tumor-related panel, in which two missense mutations located in the ligand-binding domain of ER was found (ESR1 p.Leu536Gln and ESR1 p.Tyr537Asn). ESR1 mutations are rare in early breast cancer at the time of diagnosis, but they have been identified to exist in up to 55% of ER-positive metastatic breast cancers that have been previously treated with antiestrogens in retrospective data sets (11). In functional modeling studies, these mutations confer constitutive ligand-independent activation of ER transcription and ERα expression, which may mediate antiestrogen resistance. In preclinical studies, breast cancer cell lines transfected with ESR1 mutations can be inhibited by higher dose fulvestrant (12); However, the patient has just progressed from the treatment of fulvestrant, so we finally took mild treatment and symptomatic treatment for the patient.
The mechanism of metastasis to the heart should be via the hematogenous or lymphatic route. The lymphatic spread tends to give rise to pericardial metastases, while hematogenous spread preferentially gives rise to myocardial metastasis. Since the secondary malignant heart tumors that are partially or totally transferred to the heart intracavity are rare, we try to explore and explain the possible reason for this infrequent metastasis at the genome level. Except for ESR1 mutations, some mutations in the DNA repair pathway were detected, which might have led to the genome instability. ATM p.Glu1169fs mutation was one of the particular mutations. ATM is thought to be one of the master controllers of cell cycle checkpoint signaling pathways that are required for cell response to DNA damage and for genome DNA damage, and whose suppression results in the accumulation of DNA errors and genomic instability (13). This also leads to a high mutation burden and may cause cancer cells to metastasize in the cardiac intracavity. Therefore, genomic instability caused by mutations in the ATM gene may be one of the causes of cardiac metastasis, but further evidence is needed.
Even though we have performed surgical resection of the tumor and performed NGS to try to improve the patient's symptoms, prolong the patient's survival, and find a potential targeted treatment, no suitable targeted therapy has been found, unfortunately. The patient's symptoms were not satisfactorily relieved and required continuous support treatment; the patient died six months later.
In cases such as this one, the clinicians should be alert to the possibility of cardiac metastasis especially with clinical symptoms, even though there are no guidelines or standards for the diagnosis and treatment of heart metastasis. Besides typical related symptoms and signs, the effective accessory examinations are echocardiography, CT, or even PET-CT.
Genome analysis has improved our understanding of the molecular mechanisms of cancer development, metastasis to the heart or the other tissues, as well as the resistance mechanism to antineoplastic drugs. ESR1 mutations were detected after long-term endocrine therapy and may be associated with secondary resistance to endocrine drugs. Mutations in the DNA repair pathway could lead to the instability of cancer cells, which may increase the possibility of heart metastasis. Although surgery may relieve symptoms, and obtain pathology and molecular biological information, clinicians need to pay attention to potential secondary effects.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Reynen K, Kockeritz Y, Strasser RH. Metastases to the heart. Ann Oncol 2004;15:375-81. [Crossref] [PubMed]
- Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007;19:748-56. [Crossref] [PubMed]
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013;128:1790-4. [Crossref] [PubMed]
- DeFilippis EM, Nohria A, Burstein HJ, et al. A Breath-Taking Diagnosis. N Engl J Med 2019;380:81-7. [Crossref] [PubMed]
- Engberding R, Daniel WG, Erbel R, et al. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. European Cooperative Study Group. Eur Heart J 1993;14:1223-8. [Crossref] [PubMed]
- Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219-28. [Crossref] [PubMed]
- Debourdeau P, Gligorov J, Teixeira L, et al. Malignant cardiac tumors. Bull Cancer 2004;91 Suppl 3:136-46. [PubMed]
- Yi JE, Yoon HJ. Cardiac and Pericardial (18)F-FDG Uptake on Oncologic PET/CT: Comparison with Echocardiographic Findings. J Cardiovasc Imaging 2018;26:93-102. [Crossref] [PubMed]
- Bruce CJ. Cardiac tumours: diagnosis and management. Heart 2011;97:151-60. [Crossref] [PubMed]
- Ya'qoub L, Larson S, Deedy M, et al. Treatment of recurrent isolated right atrial metastatic cavitary mass from breast cancer with radiation therapy: A case report and review of literature. Echocardiography 2018;35:1680-3. [Crossref] [PubMed]
- Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 2014;20:1757-67. [Crossref] [PubMed]
- Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013;45:1446-51. [Crossref] [PubMed]
- Prokopcova J, Kleibl Z, Banwell CM, et al. The role of ATM in breast cancer development. Breast Cancer Res Treat 2007;104:121-8. [Crossref] [PubMed]