Preoperative 3D-CT bronchography and angiography facilitates single-direction uniportal thoracoscopic anatomic lobectomy
Introduction
A surgical approach should be safe, efficient, and easily learned by surgeons. It is estimated that competency in performing video-assisted thoracoscopic surgery (VATS) lobectomy could be achieved after 50 cases (1). The learning curve of uniportal VATS (UVATS) right upper lobe (RUL) lobectomy is steep, as it is considered to be the most complicated and challenging procedure due to the variation rate of the vessels. The accurate identification of RUL venous variations is helpful to anatomic resection. Previous lung anatomy is normally studied using chest X-rays and contrast-enhanced computed tomography (CT) images. Three-dimensional (3D) digital anatomy models could be used in postgraduate teaching and surgical planning (2). Several reports have described the variation and frequency of pulmonary artery (PA) and vein branches by 3D-CT bronchography and angiography (3D-CTBA) (3). The optimal anatomic approach depends on the variations of pulmonary vessels, which could be revealed using 3D-CTBA, leading to faster operation with diminished intraoperative blood loss, as compared with those without 3D-CT simulation (4). Anatomic digital models are also useful in training for inexperienced surgeons.
It is reported that variant types are more common on the right side (32.8%) than the left (2.6%), without variations in the left lower lobes (5). Briefly speaking, pulmonary vein (PV) branches are defined as (I) an anterior vein (V. Ant.) which originates from V1b and descends down the anterior side of the RUL bronchus, finally draining into the superior PV, and (II) central vein (V. Cent.) which originates from the V2a and descends through the center of the upper lobe, between B2 and B3, finally draining into the superior PV (5). In this study, we analyzed the drainage variations of RUL veins and investigated the learning curve of single-direction UVATS RUL lobectomy assisted with 3D-CTBA.
Methods
The patients who underwent anatomic resection of RUL by the same surgeon (Dr. Zhang) following preoperative 3D-CTBA simulation at Thoracic Department of Xuzhou Central Hospital between January 2017 and April 2019 were retrospectively reviewed.
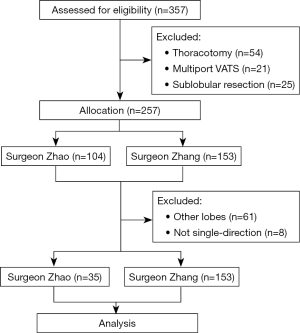
The inclusion criteria were as follows: (I) the tumor was localized without distal metastasis confirmed by whole-body contrast-enhanced CT, brain magnetic resonance imaging, and bone emission CT; (II) patients were tumor stages I–III according to the 8th AJCC/UICC tumor-node-metastasis (TNM) staging system for lung cancer (6); and (III) the American Society of Anesthesiologists score and indicators of cardio-pulmonary function such as left-ventricular ejaculation fraction, forced expiratory volume in one second, and maximal voluntary ventilation were appropriate for general anesthesia and lobectomy. Cases where the tumor or involved lymph node caused RUL atelectasis or compression of hilum structures were excluded. Segmentectomies, sleeve resections, and pneumonectomies were regularly performed by open procedure because the surgeons involved in this study lacked the experience of complex UVATS procedures. Furthermore, patients with previous thoracic operations or other cancer history, extended resections, and segmentectomy or wedge resection, were also excluded. The flow chart of this study is illustrated in Figure 1.
In addition, RUL lobectomies without 3D-CTBA performed by another surgeon (Dr. Tian Zhao) were enrolled as a control group. Surgeon Zhao had finished the learning curve of single-direction UVATS lobectomy in a high-volume center (Shanghai Pulmonary Hospital, Shanghai, China) after a year. The earlier surgical experience of the two surgeons was also evaluated to identify any difference in terms of the learning curve.
This study was approved by the Ethics Committee of Xuzhou Central Hospital (ethical approval number: XZXY-LJ-20160115-014). Informed consent was obtained from each involved patient. The data in this research were treated anonymously to maintain patient privacy.
Enhanced recovery protocol
Fast-track procedures for thoracic surgery were administered individually. Pulmonary rehabilitation was conducted regularly for 5–10 days. The clear fluid of maltodextrin solution was administered orally 6 h (400 mL) and 2 h (200 mL) before surgery. Additionally, multimodal analgesia using patient-controlled analgesia in combination with ultrasound-guided serratus anterior plane block was applied to improve pain relief after surgery. Early mobilization out of bed and direct oral feeding were started 6 h after the surgery. Moreover, prophylactic enoxaparin was started 48 h after the surgery for selected patients with Caprini score for risk assessment of venous thromboembolism ≥5.
Preoperative surgical simulation
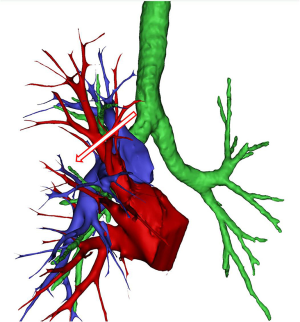
As for the surgeon in the 3D-CTBA group, contrast-enhanced thin-slice CT sections (1.0 mm thickness) were collected, and then the 3D-CTBA of anatomic variations in RUL was recorded by the free software, OsiriX. The PAs, veins, and bronchi were reconstructed and marked out with different colors, and preoperative simulation of anatomical lobectomy was performed individually based on the digital models (Figure 2) to diminish unplanned injury of abnormal vessels. Briefly, we recorded and classified RUL vein drainage into common and uncommon types.
Procedures of thoracoscopic lobectomy
All surgeons used a similar operative technique. Each patient was placed at a left-side lateral decubitus position. General anesthesia with double-lumen intubation and one-lung ventilation was administered. The surgical incision was made without rib spreading, and soft tissue retractors were used. Single-direction UVATS was performed according to the preoperative resection simulation of pulmonary vessels, with an incision of 3.5–4.5 cm as required by the expected size of the lobe in the 3rd–5th intercostal space along the anterior axillary line. Pulmonary vessels, bronchi, and incomplete fissures were divided sequentially with endoscopic staplers. Furthermore, the suction-assisted electrocautery sharp dissection technique was applied during the procedure (7). The RUL resection sequence was as follows: anterior apical branch of the PA, right upper bronchus, posterior ascending branch of the PA, PV and fissure, and the removal of the specimen using a surgical glove. The mediastinal lymph nodes were harvested after that. A 26 French chest tube was inserted for postoperative drainage. Finally, ultrasound-guided serratus anterior block was performed at the level of the 3rd–5th ribs, using 0.2% bupivacaine at a dosage of 0.3 mL per kilogram of body weight for postoperative pain relief (8).
The operation time, intraoperative blood loss, stations and numbers of harvested lymph nodes, the incidence of conversion to multiport VATS or thoracotomy, thoracic tube retention for drainage, complications defined under the Clavien-Dindo system, pain score, and postoperative hospital stay were analyzed. The patients in this cohort were followed up regularly using smartphone online, and unplanned readmission such as late-onset chylothorax or fistula was recorded.
Statistics
Continuous data were presented as means ± standard deviations (SDs). Statistical analysis was performed using Statistical Package for the Social Sciences software version 23.0 for Windows (SPSS Inc., Chicago, IL, USA). Student’s t-test or Wilcoxon test was used to compare quantitative continuous data. Chi-square or Fisher’s exact test was used when required for dichotomous or categorical variables. A P value of less than 0.05 was considered statistically significant.
Results
Baseline demographics of the patients and the surgeons
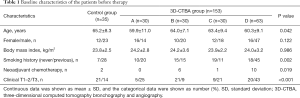
For the 3D-CTBA group, the data of 153 patients who underwent UVATS RUL lobectomy was collected, including 99 man and 54 women, with a mean age of 61.6 years. They were further divided into group A (30 cases), B (30 cases), C (30 cases), and D (63 cases), in accordance with the order of surgery. Meanwhile, the first 35 cases of single-direction UVATS right upper lobectomy by the experienced surgeon Zhao who did not use 3D-CTBA were enrolled as a control group (Table 1).
Full table
The significant difference was indicated among the 5 groups in terms of age, smoking history, neoadjuvant chemotherapy, and T staging of the tumors (P<0.05, respectively). As shown in Table 2, the previous experience of thoracotomy, multiport VATS, and UVATS anatomic, as well as the surgical volume, in the 5 years before this study of surgeon Zhao was better than surgeon Zhang. Furthermore, Zhao had finished the learning curve of single-direction UVATS lobectomy in a high-volume center (Shanghai Pulmonary Hospital, Shanghai, China). Meanwhile, during the study period, UVATS lobectomy other than RUL was performed by surgeon Zhao, while surgeon Zhang performed the RUL surgery for competency training.

Full table
Variations of the RUL veins
The digital models of the 153 patients in 3D-CTBA group revealed that there were 29 cases (19.0%) showing anomalous posterior segmental PV of RUL (V2) drainage, while the other 124 patients (81.0%) indicated the central type (V2a. Cent.). Of the uncommon RUL V2, they could be further classified into 4 types [V2a. Post. (5/153, 3.3%), VX2a. Ant. (17/153, 11.1%), VXX2a. Ant. (3/153, 2.0%), and nonspecific complicated (4/153, 2.6%)], as depicted in Figure 3.

Operative features
The patients in this cohort underwent single-direction UVATS RUL lobectomy with lymph node sampling or dissection successfully. No perioperative mortality, conversion to thoracotomy, the addition of incisions, major bleeding, lung infarction, or 30-day unplanned readmission was observed in this cohort, except for one case in group B reporting an injury of the bronchial artery which was controlled quickly.
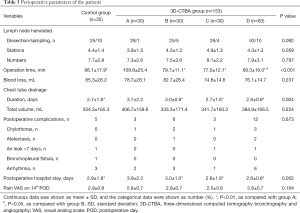
As shown in Table 3, the operation time of group A (109.8±25.4 min) was significantly longer than that of group B (79.7±11.1 min), C (77.0±12.1 min), D (69.3±16.0 min), and the control (86.1±17.9 min, P<0.001 respectively). Moreover, the operation time of the patients in group B, C, and D was slightly shorter than the control, without significance (P>0.05, respectively).
Full table
The total incidence rate of complications in the 3D-CTBA group was 15.7% (n=24), without bronchopleural fistula, empyema, or right middle pulmonary torsion, while the complication rate in the control group was 14.3% (n=5), which were controlled efficiently. All cases had an R0 complete cancer resection on histology.
Furthermore, the duration of chest tube drainage in group A (3.7±2.2 days) was noticeably longer than that in group B (3.0±0.9 days), C (2.7±1.6 days), D (2.6±0.8 days), and the control (2.7±1.6 days, P=0.004 among the groups). Similarly, postoperative hospital stay in group A (3.9±2.3 days) was noticeably longer than that in group B (3.0±1.0 days), C (2.8±1.8 days), D (2.6±0.8 days), and the control (2.8±1.8 days, P=0.002 among the groups). The 5 groups indicated comparable stations and numbers of the harvested lymph nodes, intraoperative blood loss, postoperative total chest drainage volume, incidence of complications, and pain scale on the 14th day after surgery (P>0.05, respectively).
The operation time during the learning curve of single-direction UVATS lobectomy with and without preoperative simulation is shown in Figure 4. The cases in 3D-CTBA group showed a tendency of decreased operation time, as compared with the control. Although the surgeon in the control group had finished the learning curve of single-direction UVATS lobectomy in a high-volume center before this study, the operation time of each RUL lobectomy remained unstable. It can theoretically be ascribed to a lack of a preoperative resection plan, as the surgeon should always be cautious when manipulating vessels to diminish major bleeding, which may hamper the fluency of the UVATS procedure. Based on these findings, preoperative 3D-CTBA facilitates single-direction UVATS lobectomy with a learning curve of 30 cases.

The postoperative course of the patients in this cohort was largely uneventful, and they were discharged after the removal of chest tubes. During the follow-up, they reported satisfactory quality of life and tolerable postoperative pain.
Discussion
VATS is the preferred procedure as compared with open surgery (9). Meanwhile, the predictive factors for vascular injuries and conversion during VATS partly depend on the surgeons’ experience (10). With continued experience and optimized technique, VATS lobectomy can be performed in most of the clinical stage II and IIIA non-small cell lung cancer (NSCLC) cases without compromising the oncologic efficacy including disease-free survival and overall survival of locally advanced NSCLC patients as compared with thoracotomy (11). However, a learning curve defining attainment and maintenance of proficiency in VATS lobectomy is yet to be elucidated. Most high-volume centers have demonstrated proficiency after 50 cases, while maintenance of proficiency is not ensured (12). A consensus report from the UVATS Interest Group of the European Society of Thoracic Surgeons shows that, 50 cases are required to overcome the learning curve for training in UVATS lobectomy (13), and 40 cases should be performed annually to maintain uniportal operative skills, although a detailed guideline is lacking because of the heterogeneity of different surgeons. Moreover, intensive training of young surgeons at high-volume centers may improve VATS proficiency in a short period, and provide a time-efficient modality for thoracic surgical training (14). Our preliminary study shows that the competency of single-direction UVATS RUL lobectomy assisted with 3D-CTBA could be reached after 30 cases of practice. Accordingly, there are several issues that need to be further elucidated.
First, competency in UVATS lobectomy can be acquired safely with adequate training in selected cases, although it will be achieved faster in surgeons with the earlier competency in multiport VATS lobectomy (1). However, previous surgical training has a minimal impact on intraoperative and postoperative outcomes, except conversion rate (15). Similarly, one review shows that surgeons with limited experience in open lobectomy can achieve good outcomes in VATS lobectomy comparable with their experienced seniors (16). It is noteworthy that the outcomes for lung cancer surgery are currently measured by perioperative morbidity and mortality, but the oncologic efficacy is reflected by long-term survival. Therefore, lung cancer surgery performance metrics should assess the safety of surgery and long-term survival (17).
Second, intraoperative misunderstandings of pulmonary venous anatomy can lead to serious complications such as major bleeding and delayed lung infarction or even necrosis. Furthermore, PVs play a key role as the triggering focus of the electrical activity in atrial fibrillation (18,19). However, CT scanning is still the mainstay of conventional preoperative imaging. A study evaluated the ability of different imaging techniques, such as CT scanning, maximal intensity projection imaging, 3D reconstruction, and 3D printing, to define the anatomy of hilar structures before anatomic lung resection. 3D printing in the planning of thoracic surgery may suggest a benefit over normal CT images and digital reconstruction (20). The 3D-CTBA models for precise simulation allow us to fully comprehend the vascular anatomy within a few minutes before UVATS, which is essential to avoid injury and congestion of residual lungs. As for this cohort, 29 cases (19.0%) of anomalous RUL posterior segmental PV (V2) have been revealed by 3D-CTBA. Available reports about the variations in the PV for VATS are somewhat limited, as shown in Table 4. Anomalous drainage of right V2 occurs in 7.5% of cases (5). The V2 might drain into V6 (29), and inferior PV (30). Fourdrain et al. reported a right-sided pulmonary venous variation of 36% in 100 patients, and the most frequent variation is three separate PVs (16%) (21). Yamada et al. recorded 5.8% patients (5/86) with anomalous PV and one of the variations was right-sided V2 draining directly into the left atrium (22).
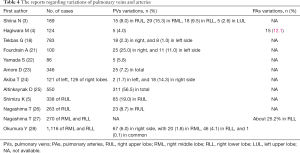
Full table
Third, there is a learning curve of the UVATS procedure to overcome before becoming proficient, which includes operative time, blood loss, number of dissected mediastinal lymph nodes and nodal stations, thoracotomy conversion rate, surgery-related complications, duration of chest drainage, postoperative hospital stay, disease-free survival, and long-term survival. However, VATS lobectomy has a vaguely defined learning curve for competency and proficiency. Li et al. reported that 100–200 cases are required to achieve efficiency, while consistency requires even more cases (31). Both UVATS lobectomy and sleeve resections for locally advanced lung tumors have a steep learning curve after proper training (32,33). Accurate 3D-CTBA anatomy models for resection simulation might also facilitate UVATS approach for bronchial, bronchovesicular, tracheal, and carinal reconstruction. The presented study indicates that the learning curve of single-direction UVATS lobectomy using 3D-CTBA for selected patients without complex manipulation is 30 cases. As compared with another experienced surgeon who did not use 3D-CTBA in this cohort, the less experienced surgeon had a tendency of shorter operation time when performing single-direction UVATS assisted with preoperative resection simulation using 3D-CTBA digital anatomic models.
Moreover, the single-direction procedure could shorten operation time to the utmost and, therefore, diminish the stress injury to the patients during surgery and anesthesia. Single-direction thoracoscopic lobectomy is characterized by incisions convenient for the placement of instruments, and the lobectomy proceeded progressively from superficial to deep structures of the thorax. It overcomes the difficulty in manipulation of incomplete pulmonary fissures and potentially extends the indications of UVATS lobectomy (34). Simplified synchronous disconnection of PAs and PVs is an effective therapeutic procedure for RUL carcinoma (35). It has been reported that non-grasping en bloc mediastinal lymph node dissection is a superior approach to remove lymph nodes, which could lead to better survival as compared with traditional grasping lymph node dissection (36).
On the other hand, it is of vital importance to reduce the risk of tumor dissemination during surgery, and a multicenter, randomized clinical trial indicates that ligating effluent veins first during pulmonary resection may reduce the dissemination of the tumor cells, and improve survival outcomes in NSCLC patients (37).
Furthermore, one of the major concerns of the learning curve is whether lymph node sampling or dissection is necessary and can be achieved sufficiently using UVATS. The number of sampled lymph nodes, one of the surrogates for quality and accurate staging, is actually influenced by many factors. The number of sampled lymph nodes has an effect on survival of NSCLC patients (sampling <10 lymph nodes versus ≥10), but the influence is dependent on staging, moreover, the optimal number of harvested lymph nodes remains unclear (38). A meta-analysis suggests that mediastinal lymph node sampling can get similar outcomes as lymphadenectomy in stage I NSCLC regarding 1-year survival, but a radical dissection of the lymph nodes is superior to sampling for 3- and 5-year survival (39). Also, for patients with tumors larger than 3 cm or N1 disease, mediastinal lymph node dissection is recommended (40). However, no consensus exists regarding the minimal or optimal number of lymph nodes to resect at curative lung cancer surgery. Research using a large database revealed that ≥16 examined lymph nodes may lead to improved survival and reduced disparities in care (41).
Similarly, another study using large databases shows that a greater number of examined lymph nodes are associated with more-accurate node staging and better long-term survival of resected NSCLC, and at least 16 lymph nodes are recommended for evaluating the quality of lymph node examination and prognostic stratification (42). As for our study, the harvested number of lymph nodes is not enough according to the number of dissected stations, and there are many reasons for this drawback. The lymph nodes were sampled or dissected according to the T staging. Also, the number of lymph nodes in the sampled or dissected soft tissues in small size might be omitted as normal fat tissue in our hospital.
This cohort study has many limitations, including but not limited to its retrospective nature and small sample size. The 3D-CTBA models which originated from CT images when the pulmonary lobes were inflated may deviate from real findings. The availability of large datasets and new deep-learning algorithms has ushered in a new era of artificial intelligence (AI) in medicine and radiology (43). AI is considered to be helpful in efficiency improvement of imaging interpretation (44). Radiographic assessment of disease most commonly relies on visual evaluations, while AI provides a qualitative interpretation of cancer imaging (45). Based on these reports, a large-scale 3D-CTBA digital anatomy database for patients undergoing accurate lobectomy is necessary for big data analysis in the era of precision medicine and AI.
Conclusions
Preoperative 3D-CTBA facilitates the safe and fluent performance of single-direction UVATS anatomic right upper lobectomy, with a learning curve of 30 cases. Well-designed, high-quality studies for better evidence are called for to verify these findings.
Acknowledgments
We appreciate doctor Tian Zhao (Department of Thoracic Surgery, East Hospital Affiliated to Tongji University, China) for his contribution in the provision of study patients and data collection.
Funding: This study is supported by Jiangsu Province Innovative and Entrepreneurial Talent Introduction Plan (No. 2-2016SC01 and 2-2016SC04).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients involved in this study for publication of any accompanying images. This study was approved by the Ethics Committee of Xuzhou Central Hospital (Ethical approval number: XZXY-LJ-20160115-014). The data in this research were treated anonymously to maintain patient privacy.
References
- Martin-Ucar AE, Aragon J, Bolufer Nadal S, et al. The influence of prior multiport experience on the learning curve for single-port thoracoscopic lobectomy: a multicentre comparative studydagger. Eur J Cardiothorac Surg 2017;51:1183-7. [Crossref] [PubMed]
- Murgitroyd E, Madurska M, Gonzalez J, et al. 3D digital anatomy modelling - practical or pretty? Surgeon 2015;13:177-80. [Crossref] [PubMed]
- Shiina N, Kaga K, Hida Y, et al. Variations of pulmonary vein drainage critical for lung resection assessed by three-dimensional computed tomography angiography. Thorac Cancer 2018;9:584-8. [Crossref] [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgerydagger. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Van Schil PE, Rami-Porta R, Asamura H. The 8th TNM edition for lung cancer: a critical analysis. Ann Transl Med 2018;6:87. [Crossref] [PubMed]
- Liao H, Liu C, Mei J, et al. Single-direction thoracoscopic lobectomy: right side. J Thorac Dis 2018;10:5935-8. [Crossref] [PubMed]
- Rahimzadeh P, Imani F, Faiz SHR, et al. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: a randomised clinical study. Turk J Anaesthesiol Reanim 2018;46:388-92. [Crossref] [PubMed]
- Ng CSH, MacDonald JK, Gilbert S, et al. Optimal approach to lobectomy for non-small cell lung cancer: systemic review and meta-analysis. Innovations (Phila) 2019;14:90-116. [Crossref] [PubMed]
- Bertolaccini L, Davoli F, Pardolesi A, et al. Conversion due to vascular injury during video-assisted thoracic surgery lobectomy: a multicentre retrospective analysis from the Italian video-assisted thoracic surgery group registry. Eur J Surg Oncol 2019;45:857-62. [Crossref] [PubMed]
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-76.e2. [Crossref] [PubMed]
- Puri V, Gaissert HA, Wormuth DW, et al. Defining proficiency for the society of thoracic surgeons participants performing thoracoscopic lobectomy. Ann Thorac Surg 2019;107:202-8. [Crossref] [PubMed]
- Bertolaccini L, Batirel H, Brunelli A, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;56:224-9. [Crossref] [PubMed]
- Sihoe ADL, Gonzalez-Rivas D, Yang TY, et al. High-volume intensive training course: a new paradigm for video-assisted thoracoscopic surgery education. Interact Cardiovasc Thorac Surg 2018;27:365-71. [Crossref] [PubMed]
- Billè A, Okiror L, Harrison-Phipps K, et al. Does previous surgical training impact the learning curve in video-assisted thoracic surgery lobectomy for trainees? Thorac Cardiovasc Surg 2016;64:343-7. [PubMed]
- Okyere S, Attia R, Toufektzian L, et al. Is the learning curve for video-assisted thoracoscopic lobectomy affected by prior experience in open lobectomy? Interact Cardiovasc Thorac Surg 2015;21:108-12. [Crossref] [PubMed]
- Fernandez FG, Kosinski AS, Tong BC, et al. Lack of correlation between short- and long-term performance after lung cancer surgery. J Thorac Cardiovasc Surg 2019;157:1633-43.e3. [Crossref] [PubMed]
- Tekbas G, Gumus H, Onder H, et al. Evaluation of pulmonary vein variations and anomalies with 64 slice multi detector computed tomography. Wien Klin Wochenschr 2012;124:3-10. [Crossref] [PubMed]
- Cheruiyot I, Munguti J, Olabu B, et al. A meta-analysis of the relationship between anatomical variations of pulmonary veins and atrial fibrillation. Acta Cardiol 2019.1-9. [Crossref] [PubMed]
- Smelt JLC, Suri T, Valencia O, et al. Operative planning in thoracic surgery: a pilot study comparing imaging techniques and three-dimensional printing. Ann Thorac Surg 2019;107:401-6. [Crossref] [PubMed]
- Fourdrain A, De Dominicis F, Bensussan M, et al. Three-dimensional computed tomography angiography of the pulmonary veins and their anatomical variations: involvement in video-assisted thoracoscopic surgery-lobectomy for lung cancer. Folia Morphol (Warsz) 2017;76:388-93. [Crossref] [PubMed]
- Yamada S, Suga A, Inoue Y, et al. Importance of preoperative assessment of pulmonary venous anomaly for safe video-assisted lobectomy. Interact Cardiovasc Thorac Surg 2010;10:851-4. [Crossref] [PubMed]
- Amore D, Casazza D, Imitazione P, et al. Common and uncommon variations of pulmonary venous drainage in patients undergoing thoracoscopic lobectomy. Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [PubMed]
- Akiba T, Marushima H, Odaka M, et al. Pulmonary vein analysis using three-dimensional computed tomography angiography for thoracic surgery. Gen Thorac Cardiovasc Surg 2010;58:331-5. [Crossref] [PubMed]
- Altinkaynak D, Koktener A. Evaluation of pulmonary venous variations in a large cohort: multidetector computed tomography study with new variations. Wien Klin Wochenschr 2019;131:475-84. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Okumura Y, Suzuki M, Takemura A, et al. Radioanatomical study of the bronchovascular anomalies of the middle and lower lobes of the right lung using multidetector computed tomography. J Comput Assist Tomogr 2009;33:529-34. [Crossref] [PubMed]
- Aragaki M, Iimura Y, Yoshida Y, et al. Anomalous V2 of the left pulmonary vein detected using three-dimensional computed tomography in a patient with lung cancer: a case report. Int J Surg Case Rep 2017;37:208-10. [Crossref] [PubMed]
- Ishikawa Y, Iwano S, Usami N, et al. An anomalous segmental vein of the left upper lobe of the lung: preoperative identification by three-dimensional computed tomography pulmonary angiography. Interact Cardiovasc Thorac Surg 2012;15:512-3. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Nachira D, Meacci E, Porziella V, et al. Learning curve of uniportal video-assisted lobectomy: analysis of 15-month experience in a single center. J Thorac Dis 2018;10:S3662-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resectionsdagger. Eur J Cardiothorac Surg 2016;49 Suppl 1:i6-16. [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Lin JB, Qiu ML, Lin CJ, et al. Simplified synchronous disconnection of pulmonary arteries and veins for right upper lobectomy. Surg Endosc 2019;33:2015-23. [Crossref] [PubMed]
- Guo C, Xia L, Mei J, et al. A propensity score matching study of non-grasping en bloc mediastinal lymph node dissection versus traditional grasping mediastinal lymph node dissection for non-small cell lung cancer by video-assisted thoracic surgery. Transl Lung Cancer Res 2019;8:176-86. [Crossref] [PubMed]
- Wei S, Guo C, He J, et al. Effect of vein-first vs artery-first surgical technique on circulating tumor cells and survival in patients with non-small cell lung cancer: a randomized clinical trial and registry-based propensity score matching analysis. JAMA Surg 2019;154:e190972. [Crossref] [PubMed]
- David EA, Cooke DT, Chen Y, et al. Does lymph node count influence survival in surgically resected non-small cell lung cancer? Ann Thorac Surg 2017;103:226-35. [Crossref] [PubMed]
- Dong S, Du J, Li W, et al. Systematic mediastinal lymphadenectomy or mediastinal lymph node sampling in patients with pathological stage I NSCLC: a meta-analysis. World J Surg 2015;39:410-6. [Crossref] [PubMed]
- Erickson CJ, Fernandez FG, Reddy RM. Minimally invasive and open approaches to mediastinal nodal assessment. Ann Surg Oncol 2018;25:64-7. [Crossref] [PubMed]
- Becker DJ, Levy BP, Gold HT, et al. Influence of extent of lymph node evaluation on survival for pathologically lymph node negative non-small cell lung cancer. Am J Clin Oncol 2018;41:820-5. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Savadjiev P, Chong J, Dohan A, et al. Demystification of AI-driven medical image interpretation: past, present and future. Eur Radiol 2019;29:1616-24. [Crossref] [PubMed]
- Fazal MI, Patel ME, Tye J, et al. The past, present and future role of artificial intelligence in imaging. Eur J Radiol 2018;105:246-50. [Crossref] [PubMed]
- Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin 2019;69:127-57. [PubMed]