
The influence of pemetrexed-based continuous maintenance therapy on survival of locally advanced and metastatic lung adenocarcinoma
Introduction
Lung cancer is the most common cancer type and is the leading cause of cancer-related death worldwide, even in China (1). The National Central Cancer Registry of China (NCCR) estimated a total of 733,300 new lung cancer cases and 610,200 lung cancer deaths in 2015 (1). Non-small cell lung cancer (NSCLC) accounts for more than 85% of new lung cancer cases, and approximately 60–70% of NSCLCs are adenocarcinomas, with most patients diagnosed with either locally advanced or metastatic disease (2).
Pemetrexed-based chemotherapy is effective and tolerable for advanced NSCLC with non-squamous histology (3,4). First-line chemotherapy with cisplatin-pemetrexed resulted in significantly longer overall survival (OS) than that with cisplatin-gemcitabine in patients with adenocarcinoma (12.6 vs. 10.9 months) and large-cell carcinoma (10.4 vs. 6.7 months) histologies (3). Advanced non-squamous NSCLC patients who do not show disease progression after induction chemotherapy also benefit from pemetrexed maintenance therapy. Switch or continuation maintenance therapy with pemetrexed showed superior progression-free survival (PFS) and OS in NSCLC patients than that with placebo (5,6).
Bevacizumab is a vascular endothelial growth factor antibody; the combination of bevacizumab and standard first-line platinum doublet treatment significantly improved survival of patients with non-squamous NSCLC in randomized phase III and phase IV trials (7-11). Moreover, the benefit of continuous maintenance therapy with bevacizumab after induction was observed on retrospective analysis of the phase 3 trial E4599 (12), phase IV trial Aries (13), and US community data (14). The phase III AVAPERL trial revealed that, compared to bevacizumab monotherapy, combination treatment with bevacizumab-pemetrexed in a maintenance setting resulted in improved PFS in patients with advanced non-squamous NSCLCs who did not show disease progression after first-line induction treatment with bevacizumab-cisplatin-pemetrexed (15).
The recombinant human endothelial inhibitor (Endostar, rh-endostatin), another antiangiogenic agent, was approved for NSCLC treatment in China in 2005. Rh-endostatin combined with chemotherapy improved the objective response rate (ORR) and time to progression in patients with advanced NSCLC, compared to chemotherapy alone (16). A trend of longer PFS was also observed in NSCLC patients receiving maintenance chemotherapy with rh-endostatin plus pemetrexed, compared to those receiving maintenance pemetrexed monotherapy (17).
In real life, patients may not receive maintenance therapy or may receive delayed maintenance therapy (stopping interval >21 days) owing to many factors. In addition, the optimal time interval between each maintenance therapy cycle is unclear. Therefore, this study aimed to assess the influence of continuous maintenance therapy with pemetrexed with or without antiangiogenesis inhibitors on survival and to evaluate the impact of prolonged interval periods on clinical outcomes.
Methods
Patients
Patients diagnosed with lung adenocarcinoma between January 2015 and December 2017 at the Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan City, Hubei Province, China, were screened. The inclusion criteria were as follows: (I) histologically or cytologically confirmed lung adenocarcinoma; (II) stage IIIB to IV disease; (III) Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0–2; and (IV) patients who received induction chemotherapy of pemetrexed-platinum (PP) with or without antiangiogenesis inhibitors (bevacizumab or rh-endostatin) every 3 weeks for 4–6 cycles and did not show progression after completion of induction chemotherapy. The exclusion criteria included: (I) squamous NSCLC, large-cell carcinoma, and other concurrent malignant tumors; (II) those who received first-line chemotherapy regimens other than PP; and (III) serious systemic diseases, such as heart failure or severe liver dysfunction. This was a retrospective observational study and patient information was collected from the hospital database. There was no research intervention for patients. This study was conducted in compliance with the Declaration of Helsinki and was approved by the hospital institutional review board (IORG No: IORG0003571).
Treatment
In our center, first-line pemetrexed-based induction therapy was administered as pemetrexed (500 mg/m2, day 1), platinum [cisplatin 75 mg/m2, days 1–3 or carboplatin area under the curve (AUC) 5, day 1], with or without antiangiogenesis inhibitors (bevacizumab 7.5 mg/kg, day 1 or rh-endostatin 7.5 mg/m2/day, days 1–14) every 3 weeks for 4–6 cycles. The initial dose was strictly based on the prescription information of each drug except for bevacizumab, for which the recommended dose is 15 mg/kg. A dose of 7.5 mg/kg was used considering the cost and comparability to the efficacy of the 15 mg/kg dose, as observed in the AVAIL Trial (8). Patients who achieved complete response (CR), partial response (PR), or had stable disease (SD) after induction were eligible for receiving pemetrexed (500 mg/m2, day 1) with or without antiangiogenesis inhibitors (bevacizumab 7.5 mg/kg, day 1 or rh-endostatin 7.5 mg/m2/day, days 1–14) as maintenance therapy. Maintenance therapy was continued until disease progression, development of unacceptable toxicity, or if the patient wished to discontinue treatment. The administered dosage could be adjusted at the physicians’ discretion.
Baseline and treatment assessments
Collected study variables included age, sex, smoking history, ECOG score, disease stag, epidermal growth factor receptor (EGFR) mutation status, anaplastic lymphoma kinase (ALK) status, brain metastases, thoracic radiotherapy and number of induction chemotherapy cycles. The tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors, version 1.1. CR, PR, and SD were calculated as the best response from the start of induction chemotherapy.
Statistical analysis
The primary endpoints of this study were PFS and OS. PFS was measured from the first day of induction chemotherapy to the date of disease progression, recurrence, or death due to any cause. OS was measured from the first day of induction chemotherapy to the date of death or to the last follow-up. PFS and OS were calculated using the Kaplan-Meier method, and comparisons between groups were analyzed using log-rank tests with 95% confidence intervals (CIs). Clinical features were compared using the chi-square test for categorical variables. Independent prognostic factors for PFS and OS were evaluated by multivariate analysis using the Cox regression model. All tests of statistical significance were 2-sided, and a P value <0.05 was considered statistically significant. All analyses were performed using SPSS Statistics (version 24.0; IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
A total of 112 patients achieved disease control after induction chemotherapy with first-line PP with or without antiangiogenesis inhibitors (bevacizumab or rh-endostatin). Median age was 59 years (range, 29–75 years), and 70 patients (62.5%) were men. Among 112 patients, 102 (91.1%) had stage IV disease whereas others had stage IIIB disease. Most patients had ECOG-PS scores of 0–1. Twenty-one patients (18.8%) had stable brain metastases that were asymptomatic or were treated with irradiation. A total of 25% and 12.5% patients had EGFR mutations and ALK positivity, respectively. Among 112 patients, 70 received maintenance therapy (maintenance group) whereas 42 did not (non-maintenance group) owing to different factors. Comparison of the main clinical characteristics between the maintenance and non-maintenance groups is shown in Table 1. Except for disease stage and EGFR mutation status, other clinical features were well balanced between the groups.
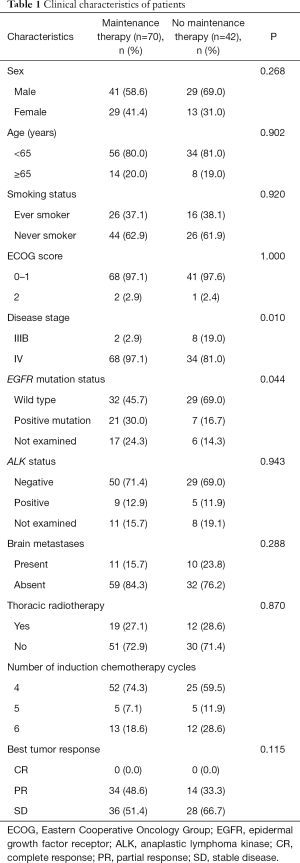
Full table
Prognostic factors
Clinical features were evaluated to determine their prognostic significance on survival. Univariate analysis showed that only lack of maintenance therapy was an adverse prognostic factor for PFS in all 112 patients (Table 2). Adverse prognostic factors for OS included the male sex, presence of brain metastases, and lack of maintenance therapy. Multivariate analysis revealed that only lack of maintenance therapy was independently associated with shorter PFS [HR, 4.516 (2.332–8.744), P<0.001]. Brain metastases [HR, 4.263 (1.499–12.127), P=0.007] and lack of maintenance therapy [HR, 4.304 (1.566–11.825), P=0.005] were independent adverse prognostic factors for OS.
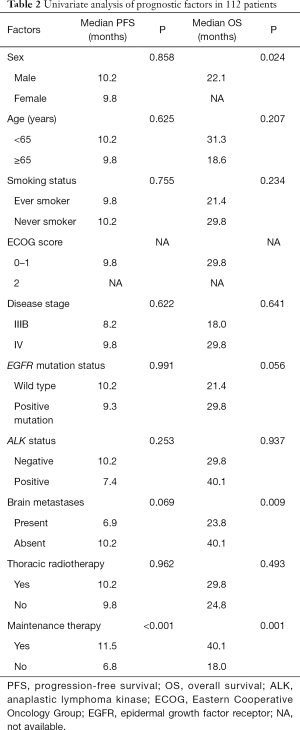
Full table
Treatment outcomes
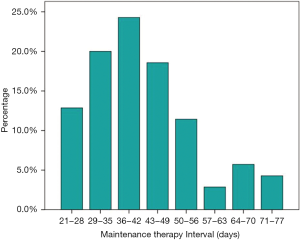
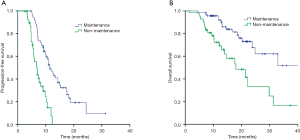
Among the 112 patients, 71 received first-line PP and 41 received PP with antiangiogenesis inhibitors (bevacizumab or rh-endostatin) as induction chemotherapy. Most patients (68.8%) received 4 cycles of induction chemotherapy. Median number of maintenance therapy cycles was 4 (range, 1–26). The interval between each maintenance therapy cycle was >35 days in 67.1% (47/70) of patients and median time interval between each maintenance therapy cycle was 40 days (range, 21–77 days, Figure 1). The best ORRs during the induction and maintenance period were 48.6% and 33.3% in the maintenance and non-maintenance groups, respectively. During a median follow-up of 14.6 months (range, 3.6–41.7 months), median PFS rates in the maintenance and non-maintenance groups were 11.5 months (95% CI: 9.8–13.2 months) and 6.8 months (95% CI: 5.4–8.2 months, P<0.001), respectively. The corresponding median OS rates were 40.1 months (95% CI: 22.5–57.7 months) and 18.0 months (95% CI: 10.4–25.6 months, P=0.001; Figure 2), respectively.
Subgroup analysis of the patients who with simple first-line PP (n=71) showed that those receiving first-line PP induction therapy followed by pemetrexed maintenance (n=40) achieved significantly longer PFS (11.2 vs. 6.8 months, P<0.001) and OS (32.9 vs. 18.0 months, P=0.006) compared with those patients without maintenance (n=31). And among patients receiving PP with antiangiogenesis induction therapy (n=41), pemetrexed with or without antiangiogenesis maintenance (n=30) also resulted in significantly longer PFS (13.4 vs. 7.0 months, P=0.008) and OS (40.7 vs. 31.3 months, P=0.057) compared with non-maintenance (n=11).
The interval between each maintenance therapy cycle ranged from 21 to 77 days. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of maintenance therapy interval. The AUC was 0.562 (P=0.406), and no optimal maintenance therapy interval was found. Using the median interval time of 40 days as the cut-off, we found that maintenance therapy intervals of ≤40 and >40 days resulted in similar PFS (11.0 vs. 12.5 months, P=0.807) and OS (40.7 vs. 37.3 months, P=0.145). Similar PFS and OS were also observed on using 35 or 42 days as the cut-off interval periods.
Driver gene mutations
We also investigated the prognosis of patients with identified tumor driver genes including EGFR and ALK. Median PFS rates in patients with EGFR mutations (n=28) and in those with wild-type EGFR (n=61) were 9.3 months (95% CI: 6.7–12.0 months) and 10.2 months (95% CI: 8.4–12.0 months, P=0.991), respectively; the corresponding median OS rates were 29.8 months (95% CI: 25.0–34.6 months) and 21.4 months (95% CI: 17.1–25.7 months, P=0.056), respectively. Median PFS rates in patients with ALK-positive tumors (n=14) and in those with ALK-negative tumors (n=79) were 7.4 months (95% CI: 4.3–10.5 months) and 10.2 months (95% CI: 8.5–11.9 months, P=0.253), respectively; the corresponding median OS rates were 40.1 months (95% CI: not applicable) and 29.8 months (95% CI: 21.0–38.6 months, P=0.937), respectively. A higher number of patients received epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and other second-line therapies after disease progression in the maintenance group than in the non-maintenance group (Table 3).
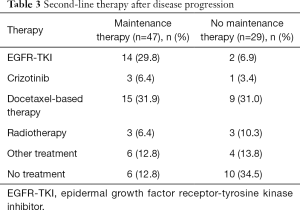
Full table
Discussion
In this study, the median time interval between each maintenance therapy cycle was 40 days. Patients with maintenance therapy administered at prolonged intervals achieved significantly longer PFS and OS compared to those without maintenance therapy. The ORR was also higher in the maintenance group than in the non-maintenance group. Consistent with the results of other studies (6,15), this study showed that continuous maintenance therapy with pemetrexed with or without antiangiogenesis inhibitors was essential for survival benefit in patients with lung adenocarcinomas who did not show disease progression after induction treatment.
In the current study, we only evaluated lung adenocarcinoma; the number of induction chemotherapy cycles was 4–6. Other studies have evaluated non-squamous NSCLCs, including lung adenocarcinoma, large-cell carcinoma, and/or bronchoalveolar tumors (6,15,18-24); the number of induction chemotherapy cycles in most studies was 4 (6,15,18,20-23). Most importantly, patients in all prospective studies received maintenance therapy every 21 days (6,15,18-23). In contrast, in this study, median interval time between each maintenance therapy cycle was 40 days. Nevertheless, despite the prolonged time interval between maintenance therapy cycles, the achieved efficacy was comparable to that obtained in previous studies (Table 4). Furthermore, similar PFS and OS were observed on using 35, 40 or 42 days as the cut-off interval periods in the maintenance group of this study.

Full table
The decision of receiving maintenance therapy usually rests with the patients and is mainly affected by their financial burden. In urban China, the average cost for lung cancer treatment is $43,336 per patient, and the financial burden in the first year of lung cancer diagnosis accounts for 171% of the household annual income (25). On estimating the direct medical and non-medical expenditure, 77.6% of families were faced with unmanageable financial burdens owing to common cancers in China (26). Moreover, 40% of lung cancer patients had limited financial reserves, i.e., reserves sufficient for less than 2 months (27). Patients with limited financial reserves experienced significantly higher pain levels, greater symptom burdens, and poorer quality of life than patients with financial reserves for more than 12 months (27). Accordingly, considering the financial burden on patients with NSCLCs, a prolonged time interval between maintenance therapy cycles seems more cost-effective.
In the present study, some patients with EGFR mutation and ALK-positivity received first-line PP-based treatment because of the following reasons. First, the EGFR and ALK status were unknown before treatment because patients chose to receive chemotherapy instead of waiting for genetic testing results. Second, a few patients could not afford EGFR-TKI treatment, as it was not covered by medical insurance before 2017. Nevertheless, among patients who received first-line PP-based treatment in this study, the median PFS was similar in patients with EGFR mutation and in those with wild-type EGFR. Even though EGFR mutation status was not similar between the maintenance and non-maintenance groups, it was not a prognostic factor for PFS. Univariate and multivariate analyses revealed that lack of maintenance therapy was the only adverse prognostic factor for PFS. However, median OS was prolonged in patients with EGFR mutation and ALK-positivity compared to patients with wild-type EGFR and ALK-negativity, although the difference was not significant. The rate of activation of EGFR mutations and number of patients who received second-line therapy after disease progression were higher in the maintenance group than in the non-maintenance group; this contributed to the long median OS observed in the maintenance group. Moreover, our results were similar to those obtained by Yoh et al. (28), who showed that 29% of patients missed second-line therapy after disease progression in the non-maintenance cohort, but only 18% of patients did not receive second-line therapy in the maintenance cohort. In addition, multivariate analysis showed that brain metastases and lack of maintenance therapy were independent adverse prognostic factors for OS.
The current study is limited by the retrospective nature and small size of the patient cohort, which may lead to selection bias. Moreover, data of some clinical characteristics of the enrolled patients were not available. To evaluate the exact value of maintenance therapy, multivariable analysis was performed to adjust for prognostic factors in this study. Further studies with larger patient cohort are needed to confirm these findings.
Conclusions
This study indicated that pemetrexed-based continuous maintenance therapy significantly improved PFS and OS in patients with lung adenocarcinomas. Lack of maintenance therapy was an independent adverse prognostic factor for both PFS and OS. The wild-type EGFR and ALK-negativity were not adverse prognostic factors for PFS in patients receiving first-line PP-based treatment. Thus, although the time interval between maintenance therapy is prolonged in clinical practice for many patients, delayed maintenance therapy still offers survival benefit in locally advanced and metastatic lung adenocarcinoma and seems to achieve similar efficacy to routine interval.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant numbers 81573090, 81773233).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the hospital institutional review board (IORG No: IORG0003571). Patient data was retrieved from hospital medical record system, so an informed consent form was not required. The patient’s personal data has been secured.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Velez M, Arango BA, Perez CA, et al. Safety and efficacy of pemetrexed in maintenance therapy of non-small cell lung cancer. Clin Med Insights Oncol 2012;6:117-24. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, Von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64-70. [Crossref] [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [Crossref] [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer: AVAiL. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Crinò L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010;11:733-40. [Crossref] [PubMed]
- Lynch TJ Jr, Spigel DR, Brahmer J, et al. Safety and effectiveness of bevacizumab-containing treatment for non-small-cell lung cancer: final results of the ARIES observational cohort study. J Thorac Oncol 2014;9:1332-9. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non–small-cell lung cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Lopez-Chavez A, Young T, Fages S, et al. Bevacizumab maintenance in patients with advanced non-small-cell lung cancer, clinical patterns, and outcomes in the Eastern Cooperative Oncology Group 4599 Study: results of an exploratory analysis. J Thorac Oncol 2012;7:1707-12. [Crossref] [PubMed]
- Kosty MP, Wozniak AJ, Jahanzeb M, et al. Effectiveness and safety of post-induction phase bevacizumab treatment for patients with non-small-cell lung cancer: results from the ARIES observational cohort study. Target Oncol 2015;10:509-16. [Crossref] [PubMed]
- Nadler E, Yu E, Ravelo A, et al. Bevacizumab treatment to progression after chemotherapy: outcomes from a US community practice network. Oncologist 2011;16:486-96. [Crossref] [PubMed]
- Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin–pemetrexed–bevacizumab for advanced nonsquamous non-small-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 2014;25:1044-52. [Crossref] [PubMed]
- Rong B, Yang S, Li W, et al. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol 2012;10:170. [Crossref] [PubMed]
- Zhou S, Zuo L, He X, et al. Efficacy and safety of rh-endostatin (Endostar) combined with pemetrexed/cisplatin followed by rh-endostatin plus pemetrexed maintenance in non-small cell lung cancer: A retrospective comparison with standard chemotherapy. Thorac Cancer 2018;9:1354-60. [Crossref] [PubMed]
- Mubarak N, Gaafar R, Shehata S, et al. A randomized, phase 2 study comparing pemetrexed plus best supportive care versus best supportive care as maintenance therapy after first-line treatment with pemetrexed and cisplatin for advanced, non-squamous, non-small cell lung cancer. BMC Cancer 2012;12:423. [Crossref] [PubMed]
- Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer. J Clin Oncol 2009;27:3284-89. [Crossref] [PubMed]
- Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol 2013;31:3004-11. [Crossref] [PubMed]
- Karayama M, Inui N, Fujisawa T, et al. Maintenance therapy with pemetrexed and bevacizumab versus pemetrexed monotherapy after induction therapy with carboplatin, pemetrexed, and bevacizumab in patients with advanced non-squamous non small cell lung cancer. Eur J Cancer 2016;58:30-7. [Crossref] [PubMed]
- Fukushima T, Wakatsuki Y, Kobayashi T, et al. Phase II study of cisplatin/pemetrexed combined with bevacizumab followed by pemetrexed/bevacizumab maintenance therapy in patients with EGFR-wild advanced non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol 2018;81:1043-50. [Crossref] [PubMed]
- Okamoto I, Aoe K, Kato T, et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naive patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs 2013;31:1275-82. [Crossref] [PubMed]
- Winfree KB, Torres AZ, Zhu YE, et al. Treatment patterns, duration and outcomes of pemetrexed maintenance therapy in patients with advanced NSCLC in a real-world setting. Curr Med Res Opin 2019;35:817-27. [Crossref] [PubMed]
- Zhang X, Liu S, Liu Y, et al. Economic burden for lung cancer survivors in urban China. Int J Environ Res Public Health 2017;14:308. [Crossref] [PubMed]
- Huang HY, Shi JF, Guo LW, et al. Expenditure and financial burden for common cancers in China: a hospital-based multicentre cross-sectional study. Lancet 2016;388:S10. [Crossref]
- Lathan CS, Cronin A, Tucker-Seeley R, et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol 2016;34:1732-40. [Crossref] [PubMed]
- Yoh K, Goto Y, Naito Y, et al. Impact of maintenance therapy for patients with non-small cell lung cancer in a real-world setting. Anticancer Res 2017;37:1507-13. [Crossref] [PubMed]


