Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial
Introduction
Video-assisted thoracic surgery (VATS) has been widely used to treat lung cancer, since it is minimally invasive, more effectively reduces postoperative pain and complications than open thoracotomy, and shortens operation time and hospital stay (1). However, postoperative pain management, particularly early postoperative pain, remains a matter of concern for several anesthesiologists and thoracic surgeons (2). Erector spinae plane block (ESPB) is an interfascial plane block that successfully deposits a local anesthetic deep into the erector spinae muscle that lies adjacent to transverse processes. Emerging research demonstrated that ESPB can be employed as a simple and safe alternative analgesic technique to address acute post-surgical, post-traumatic, and chronic neuropathic thoracic pain in adults (3) and children (4,5). Fortunately, its efficacy to ameliorate incisional pain has already been confirmed in clinical studies (6,7).
Since it is not always feasible to admit patients to a ward with indwelling peripheral nerve catheters, it is imperative to employ methods to increase the duration of analgesia with single-shot peripheral nerve blocks.
Dexmedetomidine is a potent α2 agonist and is now emerging as an adjuvant to regional anesthesia and analgesia. It can prolong the duration of the nerve block anesthesia when used with a local anesthetic, and only has a few side effects (8,9). Dexamethasone is considered to work by reducing the release of inflammatory mediators and by inhibiting potassium channel-mediated discharge of C-fibers. Results of human studies proved that the dexamethasone-treated group demonstrated longer duration of sensory and motor blockade than the control (10,11). Considering a number of studies on the efficacy of dexmedetomidine or dexamethasone as an adjuvant for ropivacaine in the erector spinae plane, we designed a double-blind randomized control study to compare the ESPB characteristics and side effects following erector spinae plane ropivacaine versus erector spinae plane ropivacaine supplemented with either dexmedetomidine or dexamethasone in patients scheduled for video-assisted thoracoscopic lobectomy surgery (VATLS).
Methods
The study was approved by the Ethics Committee of First Affiliated Hospital of Anhui Medical University (kuai 2019-02-12) and registered at clinicaltrials.gov (ChiCTR1800020041). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This randomized, controlled, double-blind study enrolled patients scheduled for lobectomy under video-assisted thoracoscopic surgery (VATS) at the first affiliated hospital of Anhui medical university (Hefei, China); all patients provided written informed consent.
All patients were aged 20–65 years, had an American Society of Anesthesiologists physical status (ASA) of I or II, and were scheduled for VATLS. The exclusion criteria were as follows: refusal to ESPB, presence of coagulopathy or bleeding disorder, bradycardia, cardiac conduction block, were administered β-adrenergic antagonist or an antiplatelet agent, local infection at the injection site, hypersensitivity to local amide anesthetics, or were hypersensitive or allergic to dexmedetomidine. Patients were also excluded if they had central neuropathy, a body mass index >35 kg/m2, uncontrolled diabetes mellitus, significant cardiopulmonary disease, or psychiatric disease.
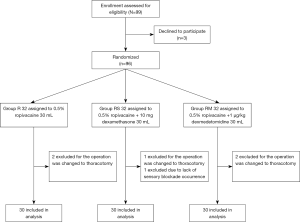
After obtaining a written informed consent, all patients were taught to evaluate their own pain by using a 10-cm visual analog pain scale (0= no pain, 10= maximum pain imaginable) and how to use the patient controlled analgesia (PCA) device at the preoperative visit. All patients were then randomized to one of three groups using computer-generated random numbers and a 1:1:1 allocation ratio. Allocation concealment was fulfilled by an assistant not involved in the study, and randomization was achieved in sequentially numbered, sealed, opaque envelopes, which were opened after patient’s arrival to the operation room. Blinding of research personnel was maintained throughout the study, including postoperative follow-ups.
Patients were placed in a standard lateral position to apply ESPB before inducing anesthesia. An assistant, who was neither involved in the study nor was participating in the perioperative period or the postoperative follow-up, prepared study drugs. Groups received 0.5% ropivacaine 30 mL (R) or 0.5% ropivacaine 30 mL with 10 mg dexamethasone (RS) or 0.5% ropivacaine 30 mL with 1 µg/kg dexmedetomidine (RM), deep to the erector spinae muscle adjacent to transverse processes.
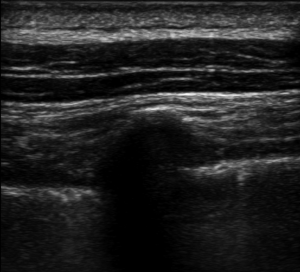
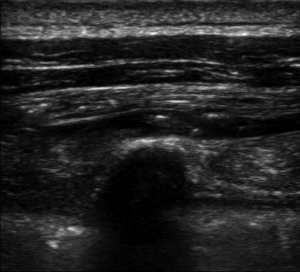
We performed ESPB in the preoperative block area following standardized monitoring, which included noninvasive blood pressure (BP), electrocardiogram (EKG), and pulse oximetry (PO). Oxygen (2–3 L/min) was supplied through the nasal cannula, and midazolam IV (0.025 mg/kg) was administered. All blocks were performed by the same three senior attending doctors with considerable experience in ultrasonic-guided nerve blocks. They were performed at the T5 level of the spine using an in-plane approach. A real-time ultrasound machine (SonoSite M-Turbo, Bothell, WA, USA) was used to evaluate block performance. A high-frequency linear ultrasound probe was placed longitudinally at a distance of 3 cm from the midline. After identifying the erector spinae muscle and transverse processes, we inserted a 22 G, 120-mm needle (stimuplex D; B. Braun Melsungen AG, Melsungen, Germany) after standard skin disinfection. It was inserted in a caudad-to-cephalad direction using a sterile probe cover until the tip lay in the interfacial plane deep into the erector spinae muscle (Figure 1). This plane was opened following hydrolocalization with normal saline. We administered 30 mL of 0.5% ropivacaine, with or without adjuvants, to ensure block performance (Figure 2). Sensory block of the 5th intercostal space in the midaxillary line was assessed by bilaterally using cold perception for 30 min after applying the nerve block. The patient was excluded from the study if sensory blockade was unsuccessful.
We connected the peripheral intravenous (IV), right internal jugular vein, and radial artery catheters while transferring the patients to the operating room. Electrocardiogram (leads II and V5), invasive blood pressure, central venous pressure, heart rate, pulse oximetry, and the bispectral index (BIS) (Vista; Aspect Medical Systems Inc., Norwood, MA, USA) were monitored throughout the procedure. Propofol (Diprivan; AstraZeneca plc, London, UK) was administered in a target-controlled infusion according to Marsh pharmacokinetic model (12) (Graseby 3500; Smiths Medical, Wat-ford, UK) while administering the anesthetic. After achieving an initial target concentration of 1.0 µg/mL, it was progressively increased by 0.3 µg/mL until the BIS value reached 40–60. Following which, 0.03 mg/kg midazolam and 0.5 µg/kg of sufentanil were intravenously injected. Rocuronium bromide (0.9 mg/kg) was used to facilitate double-lumen endobronchial intubation. After tracheal intubation, lungs were ventilated with 100% oxygen, and a volume-cycled ventilator was applied with the following settings: tidal volume of 8 mL/kg ideal body weight; inspiratory-to-expiratory ratio of 1:2; and a respiratory frequency of 8 breaths/min. Propofol and remifentanil were continuously infused to maintain anesthesia, and sufentanil and cisatracurium were administered as needed. BIS values were maintained from 40 to 60 throughout the surgery by changing the effect site concentration of propofol. The ventilation mode was switched to one-lung ventilation before the surgical procedure, and the frequency and tidal volume were adjusted to maintain pulse oximetry and end-tidal carbon dioxide. Propofol and remifentanil were discontinued upon adding the last skin suture. Neostigmine (20 µg/kg) and atropine (5–10 µg/kg) were administered according to tidal volume and frequency to reverse residual muscle relaxation at the end of surgery. Patients were admitted to the post-anesthesia care unit (PACU) until spontaneous breathing was recovered. Patients were extubated in the PACU according to standard extubation protocols and subjects were moved from the PACU on receiving a Steward recovery score of >4.
Sufentanil (0.1–0.2 µg/kg) and flurbiprofen (50 mg) were intravenously administered, followed by patient-controlled analgesia (PCA) pump use before the end of the surgery. PCA capacity was 250 mL and contained 7.5 µg/kg sufentanil and 250 mg flurbiprofen. The infusion rate was maintained at 2 mL/h, and the patient-controlled bolus was 2 mL with a lockout interval of 15 min. They were trained to press for an additional bolus if a 10 cm visual analog scale (VAS) for postoperative pain exceeded 3, and first time request for pressing PCA was recorded. In the situation when the VAS score remained ≥4 after using the PCA, the patients received tramadol 100 mg intramuscularly injection as rescue analgesic.
We performed a cold perception test in comparison with the contralateral intercostal area. Duration of sensory block was the time period from establishing the block to 100% cold perception in all sensory areas (100%= no difference to the contra-lateral side; 0%= complete sensory loss).
While under the influence of the anesthesia, mean arterial pressure (MAP) was maintained between −20% and +20% of the baseline value. A drop of 20% below the baseline MAP or a MAP<60 mmHg lasting more than 30 s was defined as hypotension. Phenylephrine (40 µg) was administered intravenously when fluid therapy was not appropriate. Atropine (0.3 mg) was administered intravenously for bradycardia, which was defined as an HR <60 bpm. Ephedrine (3–6 mg) was administered intravenously to treat bradycardia and hypotension.
The primary end point was postoperative PCA use during the first 72 h. Secondary outcomes included: (I) consumption of sufentanil, remifentanil, and propofol during anesthesia; (II) a 10 cm VAS for pain (0–10; 0, no pain; 10, worst imaginable pain) and changes in the VAS score at various time points: wake up in PACU and 2, 4, 6, 8, 12, 24, 48, 72 h after surgery; (III) optimum duration of sensory block; (IV) initial request for using PCA; and (V) incidence of postoperative nausea and vomiting (PONV) and rescue analgesia in the ward and the hospital stay after surgery.
All statistical data was analyzed primarily via SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). Calculations regarding the sample size were performed using an online power sample size calculator based on our previous pilot study showing a decreased mean effective pressing number of PCA for patients under general anesthesia combined with ESPB using ropivacaine with dexmedetomidine and ropivacaine with dexamethasone (2.6±2.2 and 3.2±2.7, respectively) compared with patients undergoing general anesthesia combined with ESPB using ropivacaine (7.4±5.0) at 72 h after surgery. To detect differences in postoperative PCA use 72 h with an SD of σ=4, the sample size was calculated as 21 per group at a power of 80% and a two-tailed α-error of 5%. We enrolled 99 patients in total (N=33/group) to countervail potential dropouts.
The Kolmogorov-Smirnov test was used to determine the normality of data distribution. The continuous variables were expressed as mean ± standard deviation, and median (25th–75th percentiles), and categorical variables as counts (percentages). We compared normally distributed continuous variables among the groups using one-way ANOVA, and used a least significant difference (LSD) procedure for post hoc comparisons, while non-normally distributed continuous variables among the groups were compared using the Kruskal-Wallis test. Mann-Whitney U tests were applied for intergroup comparisons when a significant difference was detected between the groups. Categorical variables were compared using chi-squared or Fisher’s exact test (P<0.05 was considered statistically significant).
Results
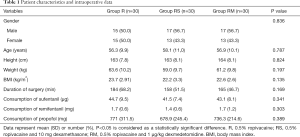
The study flow is depicted in Figure 3. Table 1 lists patient data. There was no significant difference in intraoperative characteristics among groups, which includes duration of surgery and the consumption of sufentanil, remifentanil, and propofol. Table 2 shows that postoperative VAS scores at time-points of waking up in the PACU and 2, 4, 12, 24 h after surgery in group RM decreased significantly than that in group R. Group RM demonstrated longer durations of sensory block and delayed first time of using the PCA machine than that in group R and group RS. Group RM demonstrated reduced total PCA machine use, the requirement for rescue analgesia, and postoperative hospital stay than group R and RS. There was no significant difference in the PONV occurrence rate among the groups. No patient experienced block failure, pleural effusion, subjective symptoms of local anesthetic toxicity, infection, or hematoma at the insertion site.
Full table
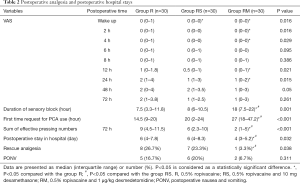
Full table
Discussion
Erector spinal plane block (ESPB) is a novel regional anesthesia technique, which is a useful intervention in thoracic neuropathic pain and acute pain after thoracic surgery or trauma (3). ESPB has been considered as a viable peripheral nerve block in establishing postoperative analgesia as it ensures greater technical simplicity, lower incidence of hypotension, and the prevention of hematoma (13). Since it is neither ideal nor feasible to admit patients to the ward with indwelling peripheral nerve catheters, there is still a need for methods to extend the analgesic effect of the single-shot nerve block postoperatively. To the best of our knowledge, this is the first controlled trial to compare the use of dexamethasone or dexmedetomidine as adjuvants to local anesthetics (LAs) for ESPB. Clinical trials have previously indicated benefits of various adjuncts to local anesthetics, but none could satisfactorily prolong effective blockade duration (14,15). We noted block time of ESPB was prolonged approximately 120% by adding perineural dexmedetomidine (1 µg/kg) to 0.5% ropivacaine. Marhofer and Kettner demonstrated that it effectively doubled the estimated clinically meaningful prolongation of peripheral nerve block (PNB) to 60% (16). Furthermore, we observed that subjects of group RM showed delay in the first time use of the PCA machine, better postoperative analgesia, and less pain intensity at all-time points with lower total PCA use during the first 72 h postoperatively than those of group R and RS. Additionally, perineural dexmedetomidine was more effective in decreasing the need for rescue analgesia and duration of hospital stay after video-assisted thoracoscopic surgery than a single injection of ropivacaine, with or without dexamethasone. Postoperative hospital stay significantly reduced in group RM, due to a notable increase in ESPB duration. Consistent with other studies (17-20), our results demonstrated that a single-injection ESPB with ropivacaine or with 10 mg dexamethasone can provide a sensory block for 7–8 h. Introducing the block in the morning or early afternoon, has led to postoperative pain during night. Opioid use may cause opioid-induced side effects, including the inhibition of restorative sleep (21) and the potential for airway obstruction and desaturation (22-24). However, a single injection of ropivacaine with dexmedetomidine can extend the sensory block to 18 h and provide a comfortable analgesia throughout the first postoperative night. Adequate and analgesic-sparing postoperative analgesia patterns are beneficial for patients to ensure their comfort, early mobilization, and reduced risks of pulmonary complications, which lead to shorter hospital stays.
Our data was similar to results of previous studies that suggested perineural dexmedetomidine may extend block duration, delayed the time for first postoperative request for PCA use, and reduced the need for postoperative rescue analgesia (25-28). A number of studies on dexmedetomidine as an adjuvant to LAs have reported that 0.5–1 µg/kg of peripheric dexmedetomidine was associated with an improved quality and duration of analgesia with no serious side effects (27). Using dexmedetomidine 100–150 µg as an adjuvant lowered the heart rate without influencing the blood pressure (29). Here, 1 µg/kg peripheric dexmedetomidine was extremely safe and effective for ASA I–II patients who underwent VATLS. Previous studies have provided possible mechanisms associated with the action of dexmedetomidine to improve blockade efficacy. First reason may be the interaction between dexmedetomidine and local anesthetics. Dexmedetomidine can cause vasoconstriction around the site of injection, which delays the absorption of the local anesthetic and prolongs the effect of the local anesthetics (30,31). Second, perineural dexmedetomidine directly affects peripheral nerve activity and attenuates acute local anesthetics-induced perineural inflammation without causing nerve damage and blocks the hyperpolarization-activated cation current (32). Finally, dexmedetomidine itself has analgesic effects and analgesic-sparing properties, and peripheral α2A-ARs were responsible for mechanisms of dexmedetomidine to treat pain in peripheral nerve block (PNB). Presynaptic α2 adrenoceptor activation inhibits the release of a transmitter from primary afferent fibers. Postsynaptic α2 adrenoceptors stimulation at the level of the spinal cord increases acetylcholine concentrations in the superficial dorsal horn and inhibits nociceptive neurotransmission by reducing the release of -neurotransmitters such as substance P and glutamate (33,34).
We did not observe any evidence of the claim that dexamethasone prolonged sensory of perineurally applied ropivacaine to the ESPB. Furthermore, VAS score, first time request for PCA machine use and total PCA use, postoperative stay in hospital and the need for rescue analgesia and PONV were not significantly different between group RS subjects, who received dexamethasone as an adjuvant to LAs, than group R. Dexamethasone did not prolong the sensory block time of ESPB in our study, similar to that reported by Marhofer and colleagues while investigating the effects of dexamethasone as an adjuvant for ulnar nerve block. However, these results seem to contradict other main evidences: perineural dexamethasone has been reported to prolong loco-regional analgesia than controls without dexamethasone (35-38). Despite precise results, using the Grading of Recommendations Assessment, Development, and Evaluation system (GRADE), Heesen and Klimek graded the quality of above evidences for these primary outcome as low (14). Since dexamethasone cannot prolong the sensory block time of ESPB as well as dexmedetomidine, similar to the results of our study, the duration and effect of postoperative analgesia have been limited.
It is a possibility that dexamethasone may act as an additive to local anesthetics and may be useful in chronic pain therapy (e.g., neuropathic pain) by attenuating the release of inflammatory mediators, reducing ectopic neuronal discharge, and inhibiting potassium channel-mediated discharge of nociceptive C-fibers (39-41).
Limitation
Similar to previous studies (42,43), 1 µg/kg peripheric dexmedetomidine was extremely safe and effective for ASA level I–II patients. However, dexmedetomidine is associated with hypotension and bradycardia (44) and patients with significant cardiovascular diseases or prone to hypotension need to be cautioned against. The pain level and sensory blockade assessment methods used were limited to subjective perception of pain and cold. Although a commonly subjective measure has been used (38,45), it does not provide objective data regarding pain and sensory blockade. We acknowledge that our work is a small randomized double-blind trial and is designed to be closely integrated with clinical applications. However, there are only a few studies that have investigated the mechanism associated with peripheral dexmedetomidine and dexamethasone in ESPB. Therefore, there is a need to investigate preclinical toxicity and clinical application to elaborate on the mechanism and the safe optimal doses of dexmedetomidine used as an adjuvant to provide a maximum benefit while minimizing side effects in peripheral nerve block (PNB).
Conclusions
Dexmedetomidine, which was used as an adjuvant of ESPB with ropivacaine, prolonged sensory block duration, provided effective acute pain control after surgery, and reduced the need for rescue analgesia. It also shortened postoperative hospital stay for patients undergoing VATLS. However, dexamethasone had no clinically relevant effect on the duration of sensory block and postoperative pain control by ropivacaine at ESPB.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of First Affiliated Hospital of Anhui Medical University (kuai 2019-02-12), all patients provided written informed consent.
References
- Grogan EL, Jones DR. VATS Lobectomy is Better than Open Thoracotomy: What is the Evidence for Short-Term Outcomes? Thorac Surg Clin 2008;18:249-58. [Crossref] [PubMed]
- Kotemane NC, Gopinath N, Vaja R. Analgesic techniques following thoracic surgery: a survey of United Kingdom practice. Eur J Anaesthesiol 2010;27:897-9. [Crossref] [PubMed]
- Forero M, Adhikary SD, Lopez H, et al. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med 2016;41:621-7. [Crossref] [PubMed]
- De la Cuadra-Fontaine JC, Concha M, Vuletin F, et al. Continuous Erector Spinae Plane block for thoracic surgery in a pediatric patient. Paediatr Anaesth 2018;28:74-75. [Crossref] [PubMed]
- Paladini G, Musella G, Farris G, et al. Erector spinae plane block to enhance recovery after thoracoscopic lung lobectomy in infants. Minerva Anestesiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Chin KJ, Malhas L, Perlas A. The Erector Spinae Plane Block Provides Visceral Abdominal Analgesia in Bariatric Surgery: A Report of 3 Cases. Reg Anesth Pain Med 2017;42:372-6. [Crossref] [PubMed]
- Restrepo-Garces CE, Chin KJ, Suarez P, et al. Bilateral Continuous Erector Spinae Plane Block Contributes to Effective Postoperative Analgesia After Major Open Abdominal Surgery: A Case Report. A A Case Rep 2017;9:319-21. [Crossref] [PubMed]
- Andersen JH, Grevstad U, Siegel H, et al. Does Dexmedetomidine Have a Perineural Mechanism of Action When Used as an Adjuvant to Ropivacaine?: A Paired, Blinded, Randomized Trial in Healthy Volunteers. Anesthesiology 2017;126:66-73. [Crossref] [PubMed]
- Rancourt MP, Albert NT, Côté M, et al. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg 2012;115:958-62. [Crossref] [PubMed]
- Choi S, Rodseth R, Mccartney CJL. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth 2014;112:427-39. [Crossref] [PubMed]
- Movafegh A, Razazian M, Hajimaohamadi F, et al. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anaesth Analg 2006;102:263-7. [Crossref] [PubMed]
- Thomson AJ, Nimmo AF, Engbers FHM, et al. A novel technique to determine an ‘apparent k e0’ value for use with the Marsh pharmacokinetic model for propofol. Anaesthesia 2014;69:420-8. [Crossref] [PubMed]
- Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med 2019;7:174. [Crossref] [PubMed]
- Heesen M, Klimek M, Imberger G, et al. Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis. Br J Anaesth 2018;120:212-27. [Crossref] [PubMed]
- Wang LZ, Liu X, Zhang YF, et al. Addition of fentanyl to the ultrasound-guided transversus abdominis plane block does not improve analgesia following cesarean delivery. Exp Ther Med 2016;11:1441. [Crossref] [PubMed]
- Marhofer D, Kettner SC, Marhofer P, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth 2013;110:438-42. [Crossref] [PubMed]
- Casati A, Fanelli G, Albertin A, et al. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol 2000;66:39-44. [PubMed]
- Hickey R, Hoffman J, Ramamurthy S. A comparison of ropivacaine 0.5% and bupivacaine 0.5% for brachial plexus block. Anesthesiology 1991;74:639. [Crossref] [PubMed]
- Hickey R, Rowley CL, Candido KD, et al. Winnie AP. A comparative study of 0.25% ropivacaine and 0.25% bupivacaine for brachial plexus block. Anesth Analg 1992;75:602. [Crossref] [PubMed]
- Vaghadia H, Chan V, Ganapathy S, et al. A multicentre trial of ropivacaine 7.5 mg•ml −1 vs bupivacaine 5 mg•ml −1 for supra clavicular brachial plexus anesthesia. Can J Anaesth 1999;46:946-51. [Crossref] [PubMed]
- Lydic R, Baghdoyan H. Neurochemical mechanisms mediating opioid-induced REM sleep disruption. In: Sleep and Pain. Wolters Kluwer Health, 2015:101-22.
- Bonafide CP, Aucutt-Walter N, Divittore N, et al. Remifentanil inhibits rapid eye movement sleep but not the nocturnal melatonin surge in humans. Anesthesiology 2008;108:627-33. [Crossref] [PubMed]
- Rosenberg J, Rosenberg-Adamsen S, Kehlet H. Post-operative sleep disturbance: causes, factors and effects on outcome. Eur J Anaesthesiol Suppl 1995;10:28-30. [PubMed]
- T Andrew B. Nocturnal arterial oxygen desaturation and episodic airway obstruction after ambulatory surgery. Anaesth Analg 2004;99:70-6. [Crossref]
- Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: A prospective randomized controlled trial. Saudi J Anaesth 2014;8:161-6. [Crossref] [PubMed]
- Rancourt MP, Albert NT, Maxime CT, et al. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg 2012;115:958-62. [Crossref] [PubMed]
- Mohamed SA, Fares KM, Mohamed AA, et al. Dexmedetomidine as an adjunctive analgesic with bupivacaine in paravertebral analgesia for breast cancer surgery. Pain Physician 2014;17:E589. [PubMed]
- Santosh BS, Mehandale SG. Does dexmedetomidine improve analgesia of superficial cervical plexus block for thyroid surgery? Indian J Anaesth 2016;60:34-8. [Crossref] [PubMed]
- Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med 2014;39:37-47. [Crossref] [PubMed]
- Yoshitomi T, Kohjitani A, Maeda S, et al. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg 2008;107:96. [Crossref] [PubMed]
- Zhang X, Bai X. New therapeutic uses for an alpha 2 adrenergic receptor agonist – Dexmedetomidine in pain management. Neurosci Lett 2014;561:7-12. [Crossref] [PubMed]
- Brummett CM, Hong EK, Janda AM, et al. Perineural Dexmedetomidine Added to Ropivacaine for Sciatic Nerve Block in Rats Prolongs the Duration of Analgesia by Blocking the Hyperpolarization-activated Cation Current. Anesthesiology 2011;115:836-43. [Crossref] [PubMed]
- Chiu KM, Lin TY, Lu CW, et al. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by α2 adrenoceptor agonist dexmedetomidine. Eur J Pharmacol 2011;670:137-47. [Crossref] [PubMed]
- Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett 2012;529:70-4. [Crossref] [PubMed]
- Parrington SJ. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med 2010;35:422-6. [Crossref] [PubMed]
- Vieira PA, Istvan P, Tsao GC, et al. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol 2010;27:285-8. [Crossref] [PubMed]
- Kim YJ, Lee GY, Kim DY, et al. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol 2012;62:130-4. [Crossref] [PubMed]
- Mao Y, Zuo Y, Mei B, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial. J Pain Res 2018;11:1811-9. [Crossref] [PubMed]
- Attardi B, Takimoto K, Gealy R, et al. Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Recept. Channels 1993;1:287. [PubMed]
- Eker HE, Cok OY, Aribogan A, et al. F250 management of neuropathic pain with methylprednisolone at the site of nerve injury. Pain Med 2012;13:443-51. [Crossref] [PubMed]
- Johansson A, Hao J, Lund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand 1990;34:335-8. [Crossref] [PubMed]
- Arunkumar S, Hemanth Kumar VR, Krishnaveni N, et al. Comparison of dexmedetomidine and clonidine as an adjuvant to ropivacaine for epidural anesthesia in lower abdominal and lower limb surgeries. Saudi J Anaesth 2015;9:404-8. [Crossref] [PubMed]
- Swami SS, Keniya VM, Ladi SD, et al. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth 2012;56:243-9. [Crossref] [PubMed]
- Carollo DS, Nossaman BD, Usha R. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 2008;21:457. [Crossref] [PubMed]
- Asida SM, Youssef IA, Mohamad AK, et al. Post-thoracotomy pain relief: Thoracic paravertebral block compared with systemic opioids. Egyptian J Anaesth 2012;28:55-60. [Crossref]