Active surveillance of ventilator-associated pneumonia in the intensive care unit and establishment of the risk grading system and effect evaluation
Introduction
Ventilator-associated pneumonia (VAP) is defined as a patient infected with pneumonia via an established artificial airway (trachea intubation or tracheotomy) and receiving mechanical ventilation including with the pneumonia occurring within 48 h of mechanical ventilation by artificial airway (1). VAP is the most common type of hospital-associated infection (HAI) (2). VAP accounts for 36–60% of all HAI cases (3) and 9–27% of ventilator-caused patient-infected VAP. The incidence rate of VAP is 1.2–8.5 per 1,000 ventilator use days (4). VAP infection can lengthen the average length of stay (LOS) to 7–9 days (5). An invasive operation can increase the risk of VAP infection, and so the intensive care unit (ICU) has the highest incidence rate of VAP among all departments. According to a recent report, about 10–20% of patients will be infected with VAP among all patients receiving invasive mechanical ventilation, and have a which crude death rate of 20–75% (6). VAP prevention and intervention including oral care (3), modified endotracheal tubes, (7) and other measures can effectively reduce a patient’s chance of contracting VAP according to existing research from other regions. Exploring the risk factors of VAP in the local region and proposing targeted preventive measures are important for local VAP control. A VAP prediction model has been developed in some studies and includes acute physiology and chronic health evaluation (APACHE) II (8,9), clinical pulmonary infection score, immunodeficiency (8,10), blood pressure, multilobular infiltrates on chest radiograph, platelets and hospitalization 10 days before onset of VAP (11), sequential organ failure assessment (10,12), simplified acute physiology score II (12), and other kinds of models. As an alternative to existing prediction models, we would like to develop a VAP risk-evaluation model based on the local situation of inpatients in the ICU. Thus, this study aimed to establish a VAP risk-evaluation model by conducting an effective evaluation based on VAP risk factor analysis which could then provide a scientific reference for clinical and HAI practitioners.
Methods
Data source
A total of 1,513 patients who received tracheal intubation or mechanical ventilation in six ICUs (general ICU, cardiovascular medicine ICU, neurology ICU, respiratory ICU, neurosurgery ICU, and cardiovascular surgery ICU) between June 2015 and June 2018, were selected in this study. All cases were randomized and divided into model samples and verification samples in a 60% and 40% balance, respectively. A total of 908 model samples were used to establish the model, and 605 verification samples were used to verify the prediction effect of the model. All patients in this study have understood and signed the informed consent form. The Ethics Board of Inner Mongolia People’s Hospital has approved this study.
Inclusion and exclusion criteria of VAP
Inclusion criteria: according to the VAP Prevention, Diagnosis and Treatment Guide published by the Intensive Care Medicine Department of the Chinese Medical Association of 2013 (13), a patient who meets the following criteria will be diagnosed as a VAP case: (I) pneumonia appears between 48 h after mechanical ventilation to 48 h after extubation; (II) new or progressive infiltrating shadow can be seen on chest X-ray image; (III) moist crackles are audible over lungs or lung solid variant syndromes appear concurrently with the appearance of one of the following conditions: (i) leucocyte level over 10.0×109/L or below 4.0×109/L, along with or not along with nuclear transfer; (ii) fever temperature over 38 °C and purulent secretion appearing in the respiratory tract; (iii) new pathogenic bacteria isolated from respiratory secretion after pneumonia infection.
Exclusion criteria: (I) the patient is already infected with VAP when admitted to ICU; (II) patient has pneumonedema, acute respiratory distress syndrome, tuberculosis, pulmonary embolism, or other related diseases.
Research method
Active surveillance was used to collect data with the Real-Time National Nosocomial Infection Surveillance (RT-NNIS) system. ICU healthcare workers (HCW) who were involved in the surveillance project were trained before the project started. Workers were trained in ventilator usage indication, VAP diagnosis standards, and VAP prevention measures. Publicity tools, including posters, handbooks, and cards, were used to let HCWs understand the purpose and method of surveillance. Once a patient was put on a ventilator, surveillance of the patient began. Each day, HAI practitioners in the Hospital-associated Infection Control Department supervised and checked patients’ condition, clinical record, nurse’s performance of infection prevention measures, and daily ICU record. VAP diagnosis judgment was jointly made by HAI practitioners and clinical doctors according to clinical symptoms and image examination. They worked together until 48 h after ventilator weaning of every patient and completion of the ventilator and infection-related information form.
Model samples were used to conduct multivariable analysis to screen possible factors. Odds ratio (OR) values of possible factors were used to establish a grading system. The receiver operating characteristic (ROC) curve was used to decide the effectiveness of the grading system. Area under curve (AUC) values between 0.5 to 1.0 were used to estimate the discriminatory power of the grading system. AUC values indicated the following: AUC closer to 1 when AUC >0.5 indicated better effectiveness of grading system; 0.5< AUC <0.7 showed a low discriminatory power; 0.7≤ AUC <0.8 showed a common discriminatory power; 0.8≤ AUC <0.9 showed a good discriminatory power; AUC ≥0.9 showed an excellent discriminatory power; AUC =0.5 showed no discriminatory power.
Indicator definition
- Tracheotomy: a tracheotomy has been performed on the research patient;
- Length of ventilator use: days of ventilator use for the research patient;
- Operation: the patient has undergone an operation in the inpatient period;
- Disorders of consciousness: the patient has a disorder of consciousness;
- Frequency of oral care: number of times per day patient has received oral care;
- Chronic disease: the patient has one or more chronic diseases;
- Subglottic secretion drainage: subglottic kind of secretion drainage has been used on a patient;
- APACHE score: APACHE II score according to Appendix D of the hospital-associated infection surveillance standard published by the Ministry of Health (14);
- Antibiotics combined use: more than one kind of antibiotics has been used on the research patient;
- Antacids use: antacids medicine has been used in the research patient.
Statistical analysis
EpiData3.0 was used to establish a database, Excel 2010 was used for data processing, and SPSS was used for statistical analysis. Case number or percentage was used to express enumeration data and conduct the χ2 test. 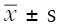 was used to express normal distributed quantitative data, and t-test was used to compare groups. Univariable analysis was conducted with the gender of the modeling group, age, APACHE II score, and other clinical data. Statistically significant factors from the univariable analysis were used to conduct multivariable logistic regression. χ2 trend tests were also performed. A P value equal to 0.05 or less was considered statistically significant in this study. ROC curve was used to evaluate the prediction effect of the grading system.
was used to express normal distributed quantitative data, and t-test was used to compare groups. Univariable analysis was conducted with the gender of the modeling group, age, APACHE II score, and other clinical data. Statistically significant factors from the univariable analysis were used to conduct multivariable logistic regression. χ2 trend tests were also performed. A P value equal to 0.05 or less was considered statistically significant in this study. ROC curve was used to evaluate the prediction effect of the grading system.
Results
Demographic and clinical information of patients
Information from the two groups including gender, age, VAP number, LOS, and other clinical data like tracheotomy, length of ventilator use, operation, disorders of consciousness, frequency of oral care, chronic disease, subglottic secretion drainage, APACHE score, combined antibiotics use, and antacids showed no significant statistical difference, meaning the two groups were comparable (Table 1).
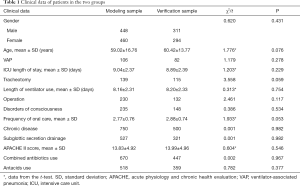
Full table
VAP infection rate
Among the 1,513 patients, 188 patients were infected with VAP for an incidence rate of 12.43% (188/1,513) and an infection rate of 15.23‰ per 1,000 days (188/12,347).
Univariable analysis of VAP patient who received mechanical ventilation in the model group
According to univariable analysis, age, LOS in ICU, mechanical ventilation days, disorders of consciousness, frequency of oral care, chronic disease, subglottic secretion drainage use, tracheotomy, APACHE II score, and antacids use, were risk factors of VAP infection of patients who received mechanical ventilation in the model group (P<0.05) (Table 2).
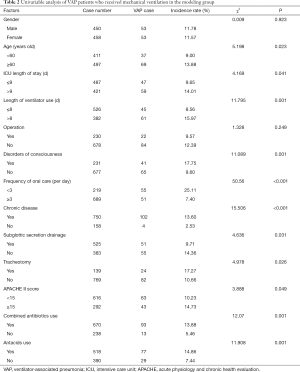
Full table
Multivariable analysis of a VAP patient who received mechanical ventilation in the model group
According to multivariable logistic regression analysis, LOS in ICU, mechanical ventilation days, frequency of oral care, unused subglottic secretion drainage, tracheotomy, APACHE II score, and combined antibiotics use were independent risk factors of VAP infection for patients who received mechanical ventilation in the modeling group (P<0.05) (Table 3).
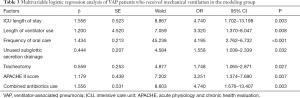
Full table
Risk grading system establishment
According to risk factors derived from the model group, factors were weighted by round-off OR value to determine the risk grade of the risk factors. The risk grade of LOS in ICU, mechanical ventilation days, frequency of oral care, tracheotomy, APACHE II score, and antibiotics combined use were 5, 3, 4, 2, 2, 3, and 5, respectively. The grading system above was used to calculate the verification group’s patient risk grade score. As determined by the approximate tripartite method, a risk grade score below 5 belonged to low risk, 6 to 10 belonged to medium risk, and above 10 belonged to high risk. The VAP incidence rate in the verification group was 13.22% (80/605), and the VAP incidence rate between the different risk grade groups showed a statistically significant difference (χ2=47.196, P<0.001). Patients with a higher-grade score had a higher risk of VAP (χ2=42.992, P<0.001) (Table 4).

Full table
Verification of risk grade system
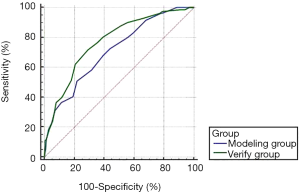
ROC curve was used to evaluate the efficiency of the risk grade system. A dependent variable of patients infected with VAP and an independent variable of patients’ risk grade score in the model group and verification group were used to draw a ROC curve. According to the curve, the AUC of the model group was 0.711, and the AUC of the verification group was 0.773, with the difference between the two groups having no statistical significance (Z=1.662, P=0.097). The AUC of the two groups were both larger than 0.7, meaning this risk grade system could effectively distinguish the risk for a patient who received mechanical ventilation and provide information for VAP prevention and treatment to the clinical practitioner (Figure 1).
Discussion
Nowadays, the incidence rate and mortality of VAP are high both in China and abroad, causing an increase in ICU LOS, length of mechanical ventilation use, and medical cost. The VAP infection rate in the U.S. is 1.9–3.8 per 1,000 days (15), and 18.3 per 1,000 days in Europe (16). VAP accounts for 36–60% of all cases of HAI (3), and among all patients receiving mechanical ventilation, about 9–27% will be infected with VAP, with a VAP infection rate of 1.2–8.5 per 1,000 days (4) leading to an increase of 7–9 days for average LOS (5). According to research in China, the average VAP infection rate is 9.52 per 1,000 days, and can vary from 4.50 to 32.79 per 1,000 days across different ICUs (17). The incidence rate of VAP in this study was 12.43% and the infection rate was 15.23 per 1,000 days, which is higher than the average level in China and the U.S., but lower than in Europe.
With a cure rate of only 2.80%, VAP is difficult to cure, and thus its mortality is high (18). Identifying the risk factors for VAP infection is urgently needed to prevent VAP infection. In this study, the risk factors with statistical significance were age, ICU LOS, length of ventilator use, a disorder of consciousness, frequency of oral care, chronic disease, unused subglottic secretion drainage, tracheotomy, APACHE II score, and antacids use (P<0.05). From the multivariable logistic regression analysis, LOS in ICU, length of ventilation use, frequency of oral care, unused subglottic secretion drainage, tracheotomy, APACHE II score, and combined antibiotics use were independent influencing factors of VAP infection. Risk factors of VAP in this study are similar to risk factors in other studies, which suggests that commonly used VAP prevention and control interventions can also be effectively implemented in the local condition (3,19).
The respiratory tract is an exchange channel between the internal and external areas of the human body which also makes it the best pathway for pathogenic bacteria from outside to invade the human body (20). The longer the length of ventilator use, the more bacteria will grow and form biofilm on the tracheal catheter. More pathogenic bacteria growing on a catheter leads to a higher risk of contracting VAP. According to this study, the chance of VAP infection will be greater the longer the mechanical ventilation use time; patient ventilator use time >8 d had a statistically significant larger incidence rate than patient ventilator use time ≤8 d (P<0.001). Therefore, a HCW should evaluate the necessity of ventilator use on a patient who is receiving mechanical ventilation; meanwhile identifying those indicators for timely extubation could reduce mechanical ventilation use time and VAP infection.
It is important for the protection of the airway that strict and effective oral hygiene care be given to patients who receive mechanical ventilation, as an artificial airway establishment, to some extent, breaks the natural protection function that the oral and nasal cavity has from pathogenic bacteria. In this way, oral care can effectively reduce the patient’s chance of getting VAP infection (3). Our results show that patients receiving oral care <3 times/d had a statistically significant higher incidence rate of VAP than those patients who received oral care ≥3 times/d (P<0.001). This indicates that sufficient provision of oral care time to patients could reduce the incidence rate of VAP.
Our results also show that patients who receive subglottic secretion drainage had a statistically lower incidence rate of VAP than those who did not receive it, which is consistent with the findings of another study (21). A possible reason for this could be that upper airway secretion gathers above the respiratory ventilator balloon, which leads to partial bacteria reproduction, whose secretion enters the lung by the airway possibly leading to lung infection. Thus, secretion drainage can effectively prevent lung infection (22).
APACHE II score is the most widely used clinical and authoritative in-critical-condition evaluation system and is helpful for predicting VAP infection, evaluating LOS, prognosis, and determining the severity of the patient. APACHE II score ≥15 means patients have a microecological imbalance, a bad immune state, and are in critical condition; patients with this score have an incidence rate statistically higher than APACHE II score <15 patients (23). According to this study, APACHE II score ≥15 was an independent risk factor of VAP, so interventions such as positive treatment of primary disease, ensuring normal physiological signs, and improving immunity, can reduce the APACHE II score and thus decrease the chance of VAP infection in turn.
Irrational use of antibiotics will generate drug-resistant bacteria and disturb the normal body flora and microbial balance leaving the body vulnerable to infection (24). Our results showed that combined antibiotic use was a risk factor. Therefore, before using antibiotics, it is advisable to administer a microbial test, choose proper antibiotics according to a drug sensitivity test, and focus on the method and concentration of the antibiotics.
All patients involved in this study were divided into the model group and verification group. The model group was used to establish the grading system. The HAI-risk-evaluation model and evaluation table were formed based on the results of the logistic regression analysis of HAI risk factors and weighing each risk factors by their OR value. Then, the HAI evaluation model and table were used to screen high HAI risk patients from inpatients. Interventions of bundle measures were administered to these high HAI risk patients, and the effects after intervention were observed. The feasibility of the HAI-risk-evaluation model in HAI prevention and control was determined based on the observations above. The verification group, on the other hand, was used to verify the grading system in order to twice ensure the effectiveness of the grading system.
The risk grade system can be used to evaluate the VAP risk to patients who receive mechanical ventilation. Targeted prevention and control measures can be aided by quantitative risk grading to be used for suspected HAI patients. Thus, the establishment of a risk grading system contributes to enhancing surveillance of ICU patients who receive mechanical ventilation. Timely grading and intervention can optimally reduce the chance of VAP infection.
There are some limitations of this study, which include the limited sample size, insufficient validation of the model, the model development, and validation using a different part of the same sample. The study design can be further improved in future studies by addressing the problems above.
Conclusions
ICU patients who received mechanical ventilation were used to establish and verify the VAP risk grading system, which is useful in predicting VAP infection and the monitoring of patients at high risk of VAP infection. The risk factors of VAP were identified by the risk grading system which performed well in determining the risk of VAP for each patient.
Acknowledgments
Funding: This research is supported by the State Key Laboratory for Infectious Disease Prevention and Control of the Chinese Center for Disease Control and Prevention (No. 2019SKLID305), the Infection Prevention and Control Research Fund Administration Commission of China Geriatric Society (No. GRYJ-LRK2018021), the Department of Science & Technology of Inner Mongolia [No. 2017MS(LH)0845], and the Health Commission of Inner Mongolia (No. 201703006).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Inner Mongolia People’s Hospital Ethics Board approved this study. All patients in this study have understood and signed the informed consent form. The study outcome will not affect the future management of patients. Patient data in this study were retrieved from the hospital medical record system and patient’s personal data have been secured.
References
- Regulation for Prevention and Control Healthcare Associated Infection in Intensive Care Unit, WS/T 509—2016 [J]. 2016. Available online: http://www.nhc.gov.cn/wjw/s9496/201701/1f9de66563304061a4fcd7f54a9399fb.shtml
- Hunter JD. Ventilator associated pneumonia. BMJ 2012;344:e3325. [Crossref] [PubMed]
- Gupta A, Gupta A, Singh TK, et al. Role of oral care to prevent VAP in mechanically ventilated Intensive Care Unit patients. Saudi J Anaesth 2016;10:95-7. [Crossref] [PubMed]
- Skrupky LP, McConnell K, Dallas J, et al. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit Care Med 2012;40:281-4. [Crossref] [PubMed]
- Afshari A, Pagani L, Harbarth S. Year in review 2011: Critical Care - infection. Crit Care 2012;16:242. [Crossref]
- Hassan ZM, Wahsheh MA. Knowledge level of nurses in Jordan on ventilator-associated pneumonia and preventive measures. Nurs Crit Care 2017;22:125-32. [Crossref] [PubMed]
- Deem S, Yanez D, Sissons-Ross L, et al. Randomized Pilot Trial of Two Modified Endotracheal Tubes To Prevent Ventilator-associated Pneumonia. Ann Am Thorac Soc 2016;13:72-80. [Crossref] [PubMed]
- Zhou XY, Ben SQ, Chen HL, et al. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int J Infect Dis 2015;30:144-7. [Crossref] [PubMed]
- Furtado GH, Wiskirchen DE, Kuti JL, et al. Performance of the PIRO score for predicting mortality in patients with ventilator-associated pneumonia. Anaesth Intensive Care 2012;40:285-91. [Crossref] [PubMed]
- Gursel G, Demirtas S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration 2006;73:503-8. [Crossref] [PubMed]
- Wiskirchen DE, Kuti JL, Nicolau DP. Acute physiology and chronic health evaluation II score is a better predictor of mortality than IBMP-10 in patients with ventilator-associated pneumonia. Surg Infect (Larchmt) 2011;12:385-90. [Crossref] [PubMed]
- Boeck L, Eggimann P, Smyrnios N, et al. Midregional pro-atrial natriuretic peptide and procalcitonin improve survival prediction in VAP. Eur Respir J 2011;37:595-603. [Crossref] [PubMed]
- Intensive Care Branch of Chinese Medical Association. Ventilator Associated Pneumonia Diagnosis, Prevention and Treatment Guide. Chinese Journal of Internal Medicine 2013;52:524-43.
- Hospital associated infection surveillance standard, WS/T 312-2009S.[J].2009. Available online: http://www.nhc.gov.cn/wjw/s9496/200904/40117.shtml
- Patrick SW, Kawai AT, Kleinman K, et al. Health care-associated infections among critically ill children in the US, 2007-2012. Pediatrics 2014;134:705-12. [Crossref] [PubMed]
- Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis 2017;36:1999-2006. [Crossref] [PubMed]
- Gao XD, Hu BJ, Cui YW, et al. A multicenter prospective monitoring on incidence of ventilator-associated pneumonia in 46 hospitals in China. Chinese Journal of Infection Control 2015;8:540-3.
- Nan L, Liu YX, Cao JG, et al. Multicenter study on risk factors for ventilator-associated pneumonia in intensive care unites. Chinese Journal of Nosocomiology 2017;13:2893-6.
- Mehta A, Bhagat R. Preventing Ventilator-Associated Infections. Clin Chest Med 2016;37:683-92. [Crossref] [PubMed]
- Chen XX, Li XJ, Liu JM, et al. Etiological characteristics of patient with ventilator-associated pneumonia and influencing factors. Chinese Journal of Nosocomiology 2017;27:4861-4.
- Geslain G, Guellec I, Guedj R, et al. Incidence and risk factors of ventilator-associated pneumonia in neonatal intensive care unit: a first French study. Minerva Anestesiol 2018;84:829-35. [PubMed]
- Wang BQ, Cao WJ, Wang Y, et al. Impact of different modes of subglottic suctions on pathogens causing ventilator-associated pneumonia. Chinese Journal of Nosocomiology 2014;24:3179-81.
- Gupta S, Boville BM, Blanton R, et al. A multicentered prospective analysis of diagnosis, risk factors, and outcomes associated with pediatric ventilator-associated pneumonia. Pediatr Crit Care Med 2015;16:e65-73. [Crossref] [PubMed]
- Liu WP, Sun DJ, Yan ZG, et al. Risk factors for ventilator-associated pneumonia. Chinese Journal of Nosocomiology 2017;27:85-7. [PubMed]



