
Platelet-derived HMGB1: critical mediator of SARs related to transfusion
An adverse reaction is an undesirable response in a patient associated with the administration blood component (BC) (1). The scientific community consents that platelets play a role in inflammatory responses (2). However, when transfusion is linked with SARs, most can be linked to an inflammatory state that is either apparent (allergy, FNHTR) or attributed by present understanding (alloimmunization) to such a state. The involvement of platelet concentrates (PC) in SARs could be related, at least in part, to the inflammatory functions of platelets acquired during storage lesions. We have previously shown an association between the levels of sCD40L, sCD62P, IL27, IL-13, sOX40L, and mitochondrial DNA in PCs and the risk for SARs (3).
Novelty, we investigated, the HMGB1 release through single donor apheresis (SDA)-platelet concentrates (SDA-PCs)—associated or not with SARs—process and during their storage. The concentration for HMGB1 in SDA-PCs (n=70, d1–d3: n=38 and d3–d5: n=32) was compared to levels of HMGB1 in SDA-PCs involved in SARs (n=78, d1–d3: n=22 and d3–d5: n=56). HMGB1 concentrations (Figure 1) were higher in SDA‐PCs which transfusions was associated with SARs in the recipients than in the matched control PCs. This was true for young PCs (1–3 days): 0.6710±0.1548 vs. 3.9970±0.6349 ng/mL (P<0.003) and old PCs (3–5 days) 0.6166±0.2187 vs. 2.4340±0.0.2640 ng/mL (P<0.0001).
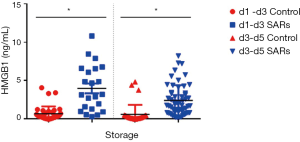
We now provide first evidence that levels of soluble HMGB1 are also strongly associated with SARs. However, patients receiving fresh PCs may develop SARs if they are susceptible to slightly increased HMGB1 levels. Future studies will attempt to evaluate the importance of the processing and of storage of PCs and to correlate with transfusion yield. Reducing accumulation of the storage lesion side product in PC might be an interesting target to reduce the risk of SARs (4).
It is obviously recognized that the enhanced concentrations of cytokines and chemokines in platelet concentrate produced during storage may contribute to SARs (5). Transfusion-linked inflammation is probably the consequence of a combination of factors related to the donor, the BC, and the recipient. The main factor that can be targeted at this moment, by the company in partnership with blood establishments, is the BC and measures to enhance BC quality independently of the inflammatory state. Identifying parameters that may be linked to donor, BC, and patients would be beneficial to define “How can a patient receive the most appropriate BC for their situation?”.
Acknowledgments
We would also like to thank the facility technical staff of the University of Saint-Etienne EA3064 GIMAP. We thank the blood donors for taking part in this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood 2019;133:1831-9. [Crossref] [PubMed]
- Kapur R, Zufferey A, Boilard E, et al. Nouvelle cuisine: platelets served with inflammation. J Immunol 2015;194:5579-87. [Crossref] [PubMed]
- Sut C, Tariket S, Aubron C, et al. The Non-Hemostatic Aspects of Transfused Platelets. Frontiers in medicine 2018;5:42. [Crossref] [PubMed]
- Frenette PS, Mohandas N. Bad blood: a trigger for TRALI. Nature medicine 2010;16:382-3. [Crossref] [PubMed]
- Cognasse F, Laradi S, Berthelot P, et al. Platelet Inflammatory Response to Stress. Frontiers in immunology 2019;10:1478. [Crossref] [PubMed]


