The PLAN score can predict poor outcomes of intracerebral hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a sudden and life-threatening event that accounts for 10–15% of all stroke cases (1,2). Mortality rates for ICH patients are as high as 46–48% and with only 32.8–42.4% of patients being able to function independently by 1 year post-ICH hospitalization (3,4).
At the time of admission, it is important to evaluate the possibility of death and severe disability to make rational treatment decisions for ICH patients. Making these decisions quickly is also important to help determine if patients are eligible for clinical trials, which will help assess the use of novel therapies in ICH. These considerations necessitate the development of a model that will help clinicians efficiently stratify patients for accurate assessment of therapeutic efficacy using varying treatment modalities and help predict the prognosis of ICH.
Several prognostic tools have been developed for ICH, including the ICH score (5), intracerebral hemorrhage grading scale (ICH-GS) (6), Essen-ICH score (7), max-ICH score (8), the simplified ICH score (sICH score) (9), ICH functional outcome score (ICH-FOS) (10), modified ICH (MICH) score (11), ICH outcomes project (ICHOP) score (12), and the functional outcome (FUNC) score (13). Even more of these prognostic tools have been developed for acute ischemic stroke (AIS), including the acute stroke registry and analysis of Lausanne (ASTRAL) score (14), preadmission comorbidities, level of consciousness (LOC), age and neurologic deficit (PLAN) score (15), ischemic stroke predictive risk score (IScore) (16), the Bologna outcome algorithm for stroke (BOAS) (17), Get with the Guidelines Stroke (GWTG-Stroke) risk model (18), totaled health risks in vascular events (THRIVE) score (19), subtype, Oxfordshire Community Project classification, age and prestroke modified Rankin (SOAR) score (20), and thrombolytic predictive instrument (TPI) score (21). In these prediction models, during the acute phase of a stroke, the most important predictors of outcome are patient age and stroke severity (22). Other important predictors of outcome are related to the presence of comorbid medical conditions and functional status prior to stroke onset (23). Predictive assessment of poor outcomes in AIS may share similarities with predicting poor outcomes in ICH. Of the models mentioned, the PLAN score is desirable because of its simplicity and integration multiple factors including preadmission comorbidities, LOC, age, and neurologic focal deficit (15). The ease of use in calculating PLAN scores may be valuable in the time-sensitive setting of ICH hospitalization. We used PLAN scoring in an analysis of ICH patients in the China National Stroke Registry (CNSR) to determine if it can help predict poor outcomes following ICH hospitalization.
Methods
Study design
We conducted this study using the CNSR, the largest stroke registry in China. The CNSR is sponsored by the Chinese Ministry of Health and is a national, multicenter, and prospective registry. The CNSR recruited patients from September 2007 to August 2008 who suffered from acute cerebrovascular disease. These patients were from 132 participating centers in 27 provinces and 4 municipalities spanning the entirety of China, including Hong Kong SAR (24). To minimize the variations among different areas due to factors such as infrastructure, hospital size, population composition, as well as maximize the geographic and demographic representativeness, participating centers were strategically chosen from the west, east, and central areas of China. The total number of patients in the CNSR is 22,216 consecutive eligible patients. Signed, written informed consent was obtained from every enrolled patient or their legal guardian.
Eligibility criteria
The cohort used in this study included CNSR patients who were ≥18 years of age with a primary diagnosis of ICH. We excluded patients diagnosed with transient ischemic attack, ischemic stroke, or subarachnoid hemorrhage. ICH diagnoses were based on the World Health Organization criteria combined with brain computerized tomography (CT) imaging. ICH patients in this study also must have presented to hospital no more than 14 days after symptom onset and be >18 years old. Exclusion criterion included tumor-induced ICH, unavailable data of ICH hematoma volume, primary intraventricular hemorrhage (IVH), >2 pre-stroke modified Rankin Scale score (mRS), patients who did not agree take part in the study, and those who were lost during follow-up. The central institutional review board at Beijing Tiantan Hospital and ethical committees at each participating center of the CNSR approved the scientific use of the registered data for this study.
Data collection
Patient demographics (e.g., age, gender) were recorded upon admission. Baseline vascular risk factors included stroke history (confirmed from a medical chart), hypertension (history of hypertension or use of antihypertensive medicine), diabetes mellitus (history of diabetes mellitus or use of hypoglycemic drug), dyslipidemia (history of dyslipidemia or use of lipid-lowering medicine), atrial fibrillation (history of atrial fibrillation based on the confirmation of at least one electrocardiogram, or onset of arrhythmia during hospitalization), cardiovascular disease, active smoking, moderate or heavy alcohol consumption (≥2 standard alcoholic beverages a day), body mass index (BMI, weight in kilograms divided by the square of height in meters), and medication history including antihypertensive, antiplatelet, anticoagulant, lipid-lowering, and hypoglycemic agents. Other risk factors such as cancer (prior or concurrent, active or metastatic) and lung disease were also noted. We used mRS to evaluate pre-stroke functional status and the National Institute of Health Stroke Scale (NIHSS) and Glasgow Coma Scale (GCS) to assess the severity of neurological impairment within 24 hours of admission. Routine laboratory tests data were recorded. To acquire information on mRS, death, and the corresponding dates of outcomes, all patients’ functional outcomes were evaluated via telephone follow-up at 3, 6, and 12 months post-ICH hospitalization. Telephone follow-up was uniform for all study subjects using a shared, standardized interview protocol administered by trained interviewers.
Statistical analyses
The calculation of patients’ PLAN score was performed as described in the original article (15). For descriptive analysis, we used percentages for categorical variable and mean with standard deviation (SD) for continuous variables. To compare categorical variables, we used a χ2 test and for the continuous variables, we used the t-test or Wilcoxon rank sum test for continuous variables. PLAN score was used to divide the CNSR cohort into six risk categories. A logistic regression model was applied for evaluation of PLAN score’s poor outcome predictive value using 30-day mortality, mRS 5–6 at discharge, and 1-year mortality. Area under the curve (AUC) from the C statistic was used to establish prediction score performance. A C statistic of 1.0 indicates a perfect prediction, whereas a C statistic of 0.5 indicates that the prediction does not differ from a random prediction. All tests were 2-tailed. P<0.05 was deemed statistically significant. SAS version 9.4 statistical software (SAS Institute Inc., Cary, North Carolina, USA) was used for all analyses.
Results
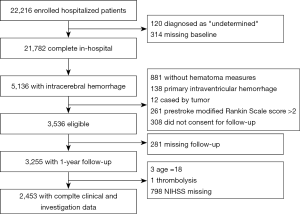
Five thousand one hundred and thirty-six of the 22,216 CNSR patients were diagnosed with ICH and screened for eligibility for this study. Using the previously described exclusion criteria, we excluded 881 patients who had no hematoma measurement data, 138 with primary IVH, 12 with ICH secondary to an intracranial tumor, 261 with a pre-stroke mRS >2,308 who did not consent for follow-up, 281 lost to follow-up, three 18-year-old patients, one patient with ICH secondary to thrombolysis, and 798 patients without NIHSS. The remaining 2,453 patients were analyzed in Figure 1.
The mean age of these ICH patients was 62.17±12.95 years and 946 (38.6%) were female. Patient demographics and clinical characteristics at baseline are summarized in Table 1.
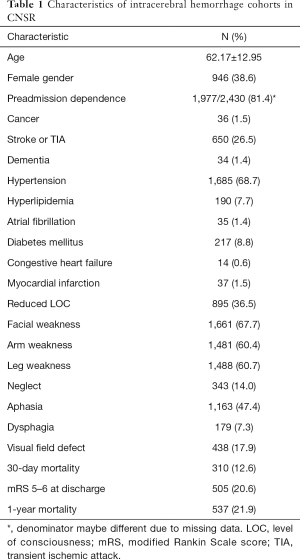
Full table
Univariate analysis showed age, preadmission dependence, reduced LOC, arm weakness, leg weakness, aphasia and/or neglect were significantly associated with 30-day mortality, mRS 5–6 at discharge, and 1-year mortality (P<0.001). Atrial fibrillation was only significantly associated with mRS 5–6 at discharge. However, cancer and congestive heart failure were not associated with mortality (Table 2).
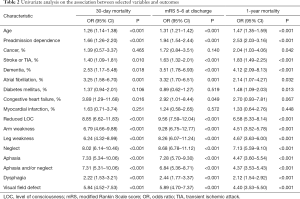
Full table
Multivariable analysis showed age, reduced LOC, aphasia and/or neglect were significantly predictive of 30-day mortality, mRS 5–6 at discharge, and 1-year mortality (Ps<0.001). Arm weakness was only significantly associated with mRS 5–6 at discharge. Pre-admission dependence, cancer, atrial fibrillation, congestive heart failure, and leg weakness were not significantly associated with mortality (Table 3).
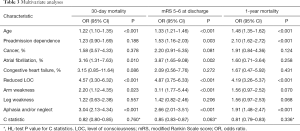
Full table
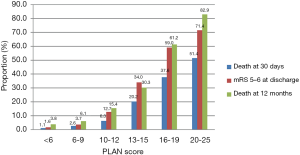
Figure 2 shows that 30-day mortality, mRS 5–6 at discharge, and 1-year mortality after ICH onset increased gradually as PLAN score increased.
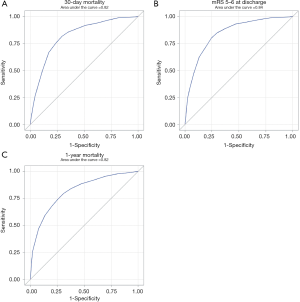
AUC values were used to estimate the accuracy of the PLAN score in predicting poor outcome. The PLAN score showed good discriminatory power with AUC values of 0.82, 0.84 and 0.82 at 30-day discharge and 1-year follow-ups, respectively (Figure 3). This was consistent even after grouping by age and gender, as the PLAN score still showed good discriminatory power as all the AUC values were still ≥0.80 (Table 4).

Full table
Discussion
The CNSR is a large-scale stroke registry well-suited for the evaluation of PLAN score predictive value in ICH patients. Using this database, we found that the PLAN score reliably predicted poor outcomes following ICH hospitalization. Our study represents the first assessment of the predictive ability of the PLAN score in ICH using a nationwide cohort.
It is well-established that stroke risk prognostic scores can improve clinician accuracy in predicting clinical outcomes (25). The PLAN score was developed in Canada (15) and validated in several AIS populations with varying ethnicities (26,27), and a Scottish mixed stroke patient cohort (88% AIS, 12% ICH) (25). In our study, we found that the PLAN score had performed well in predicting the 30-day mortality (C statistics: 0.82; 95% CI, 0.70–0.84), mRS 5–6 at discharge (C statistics: 0.84; 95% CI, 0.83–0.86) and 1-year mortality (C statistics: 0.82; 95% CI, 0.80–0.84), which is similar to the predictive ability in AIS patients (15,25-27).
In the past two decades, several prognostic tools have been developed for ICH. Hemphill et al. introduced the ICH score (5) in 2001, which were followed by a number of modified and pragmatic ICH scoring methods (6-12). The original ICH score was used to predict 30-day mortality (C statistics: 0.825; 95% CI, 0.811–0.838) and 1-year mortality (C statistics: 0.798; 95% CI, 0.783–0.811) (5). The ICH-GS score assessed 30-day mortality (C statistics: 0.830; 95% CI, 0.817–0.843) and 1-year mortality (C statistics: 0.814; 95% CI, 0.800–0.827) (6) along with the ICH-FOS score, which also predicted 30-day mortality (C statistics: 0.836; 95% CI, 0.822–0.848) and 1-year mortality (C statistics: 0.836; 95% CI, 0.822–0.848) (10). Those prognostic models use similar variables, such as age, GCS score, ICH volume, ICH location, NIHSS score, and serum glucose, along with a few variables that required a significant waiting period such as CT and detailed lab exams. Clinical grading scales must be reliable, accurate, and practical and to-date none of these prognostic tools have been universally accepted in routine clinical practice. The PLAN score, which was developed as a bedside prediction rule for death and severe disability following AIS, addresses issues of practicality and efficiency by only requiring basic information such as preadmission comorbidities, LOC, age, and neurologic focal deficit, which are all established risk factors that can be evaluated by non-specialist clinicians. The simplicity and high predictive value of the PLAN score may allow it to have widely accepted use for prediction of poor outcomes in post-hospitalization ICH patients.
Despite the proposed benefits of using PLAN in ICH patients, we acknowledge a few limitations of this study. First, only hospitalized patients were included, while those who died in the emergency department or sought treatment in the outpatient setting were not considered in our study. Second, misjudgment of patients’ functional outcome evaluation may be present because interviewees might not be able to give accurate answers during the follow-up phone interview. Although prior studies have shown that assessment of the mRS through structured telephone is reliable and comparable with face-to-face interviews (28). Third, the predictions performed at first assessment were mostly within 24 hours after ICH onset, yet predictions performed >24 hours from onset are more accurate and over the first 24 hours, the association between stroke severity and 90-day mRS strengthens (29). Despite these limitations, a reliable method of early prediction should be used to inform vital early clinical decision-making and discussions regarding prognosis with patients and relatives. Further validation of the PLAN score is needed in non-Asian ICH populations. Future work must also examine whether the PLAN score can improve prediction of ICH patient outcomes when compared to the assessment of experienced stroke-specialized clinicians.
Conclusions
The PLAN score can predict 30-day mortality, death or severe dependence at discharge, and 1-year death after ICH hospitalization. This score was developed originally for use in AIS, but it may also help identify ICH patients who could develop poor outcomes following hospitalization. Further studies are needed to validate the PLAN score for use in ICH patients from different ethnic backgrounds.
Acknowledgments
Funding: This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China [2016YFC0901002, 2016YFC0901001, 2017YFC1310901, 2017YFC1307905, 2017YFC1307702, 2018YFC1312903], grants from Beijing Municipal Administration of Hospitals’ Mission Plan [SML20150502], grants from National Natural Science Foundation of China [81600999], grants from Beijing Municipal Science & Technology Commission [D171100003017002, D151100002015003] and grants from National Science and Technology Major Project [2017ZX09304018].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The central institutional review board at Beijing Tiantan Hospital and ethical committees at each participating center of the CNSR approved the scientific use of the registered data for this study. Written informed consent was obtained from every enrolled patient or their legal guardian.
References
- Caplan LR. Intracerebral haemorrhage. Lancet 1992;339:656-8. [Crossref] [PubMed]
- Wang W, Wang D, Liu H, et al. Trend of declining stroke mortality in China: reasons and analysis. Stroke Vasc Neurol 2017;2:132-9. [Crossref] [PubMed]
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660-7. [Crossref] [PubMed]
- Dastur CK, Yu W. Current management of spontaneous intracerebral haemorrhage. Stroke Vasc Neurol 2017;2:21-9. [Crossref] [PubMed]
- Hemphill JC 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891-7. [Crossref] [PubMed]
- Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, et al. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke 2007;38:1641-4. [Crossref] [PubMed]
- Weimar C, Benemann J, Diener HC, et al. Development and validation of the Essen Intracerebral Haemorrhage Score. J Neurol Neurosurg Psychiatry 2006;77:601-5. [Crossref] [PubMed]
- Sembill JA, Gerner ST, Volbers B, et al. Severity assessment in maximally treated ICH patients: The max-ICH score. Neurology 2017;89:423-31. [Crossref] [PubMed]
- Chuang YC, Chen YM, Peng SK, et al. Risk stratification for predicting 30-day mortality of intracerebral hemorrhage. Int J Qual Health Care 2009;21:441-7. [Crossref] [PubMed]
- Ji R, Shen H, Pan Y, et al. A novel risk score to predict 1-year functional outcome after intracerebral hemorrhage and comparison with existing scores. Crit Care 2013;17:R275. [Crossref] [PubMed]
- Cho DY, Chen CC, Lee WY, et al. A new Modified Intracerebral Hemorrhage score for treatment decisions in basal ganglia hemorrhage--a randomized trial. Crit Care Med 2008;36:2151-6. [Crossref] [PubMed]
- Gupta VP, Garton ALA, Sisti JA, et al. Prognosticating Functional Outcome After Intracerebral Hemorrhage: The ICHOP Score. World Neurosurg 2017;101:577-83. [Crossref] [PubMed]
- Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304-9. [Crossref] [PubMed]
- Ntaios G, Faouzi M, Ferrari J, et al. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology 2012;78:1916-22. [Crossref] [PubMed]
- O'Donnell MJ, Fang J, D'Uva C, et al. The PLAN score: a bedside prediction rule for death and severe disability following acute ischemic stroke. Arch Intern Med 2012;172:1548-56. [Crossref] [PubMed]
- Saposnik G, Kapral MK, Liu Y, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation 2011;123:739-49. [Crossref] [PubMed]
- Muscari A, Puddu GM, Santoro N, et al. A simple scoring system for outcome prediction of ischemic stroke. Acta Neurol Scand 2011;124:334-42. [Crossref] [PubMed]
- Smith EE, Shobha N, Dai D, et al. Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With the Guidelines-Stroke Program. Circulation 2010;122:1496-504. [Crossref] [PubMed]
- Kamel H, Patel N, Rao VA, et al. The totaled health risks in vascular events (THRIVE) score predicts ischemic stroke outcomes independent of thrombolytic therapy in the NINDS tPA trial. J Stroke Cerebrovasc Dis 2013;22:1111-6. [Crossref] [PubMed]
- Myint PK, Clark AB, Kwok CS, et al. The SOAR (Stroke subtype, Oxford Community Stroke Project classification, Age, prestroke modified Rankin) score strongly predicts early outcomes in acute stroke. Int J Stroke 2014;9:278-83. [Crossref] [PubMed]
- Kent DM, Selker HP, Ruthazer R, et al. The stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke 2006;37:2957-62. [Crossref] [PubMed]
- Ntaios G, Papavasileiou V, Michel P, et al. Predicting functional outcome and symptomatic intracranial hemorrhage in patients with acute ischemic stroke: a glimpse into the crystal ball? Stroke 2015;46:899-908. [Crossref] [PubMed]
- Quinn TJ, Taylor-Rowan M, Coyte A, et al. Pre-Stroke Modified Rankin Scale: Evaluation of Validity, Prognostic Accuracy, and Association with Treatment. Front Neurol 2017;8:275. [Crossref] [PubMed]
- Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke 2011;6:355-61. [Crossref] [PubMed]
- Reid JM, Dai D, Delmonte S, et al. Simple prediction scores predict good and devastating outcomes after stroke more accurately than physicians. Age Ageing 2017;46:421-6. [PubMed]
- Wang WY, Sang WW, Jin D, et al. The Prognostic Value of the iScore, the PLAN Score, and the ASTRAL Score in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 2017;26:1233-8. [Crossref] [PubMed]
- Xu J, Tao Y, Xie X, et al. A Comparison of Mortality Prognostic Scores in Ischemic Stroke Patients. J Stroke Cerebrovasc Dis 2016;25:241-7. [Crossref] [PubMed]
- Merino JG, Lattimore SU, Warach S. Telephone assessment of stroke outcome is reliable. Stroke 2005;36:232-3. [Crossref] [PubMed]
- Saver JL, Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke 2012;43:1537-41. [Crossref] [PubMed]