
Design of stroke imaging package study of intracranial atherosclerosis: a multicenter, prospective, cohort study
Introduction
Intracranial atherosclerosis (ICAS) is one of the leading causes of stroke which occurs disproportionately among different races. Compared with extracranial atherosclerosis (ECAS), ICAS had a higher prevalence in Asian, Hispanics and African-American patients. It accounts for 30–50% stroke in Asians and Blacks while 8–10% in Whites (1,2). The onset age of ICAS also tends to be younger in Asians than Caucasians. Given the difficulties in obtaining ICAS pathological specimens in living people, evidence from extracranial and coronary arteries has often been used for reference in ICAS management (3,4). However, ICAS has unique features such as a dense internal elastic lamina (IEL), nearly no external elastic lamina (EEL) and relative paucity of vasa vasorum in adventitia (2,5). The results from stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) and Vitesse intracranial stent study for ischemic therapy (VISSIT) trials have proved that aggressive medical treatment is superior to endovascular therapy even in high-risk ICAS patients, which is different from ECAS (6,7). Therefore, it is insufficient and imprecise to treat ICAS based on indirect evidence from ECAS. Owing to the great impact of ICAS worldwide and its distinctive characteristics, there is an urgency to study ICAS further.
Currently, conventional imaging methods including magnetic resonance angiography (MRA), computed tomography angiography, digital subtraction angiography are commonly utilized to identify ICAS in clinical practice. On these vessel imaging, the luminal stenosis degree is regarded as the major indicator to evaluate severity of illness and predict stroke prognosis. However, data from several large trials demonstrated that luminal stenosis was not the only risk factor for unfavorable outcomes in ICAS patients (8). Moderate luminal stenosis could lead to fatal strokes (9,10). Therefore, it is necessary to develop a new imaging technique to provide more information of ICAS beyond the lumen. In this regard, high-resolution magnetic resonance imaging (HR-MRI) is the appropriate tool to directly depict vessel walls and exhibit pathological changes, including morphology of vessel wall (eccentricity and remodeling) and plaque features (number, volume, distribution, and components) (11-13).
Using a novel stroke imaging package including HR-MRI, the aim of this proposed cohort study is to investigate the relations between imaging features and clinical events, and set up a predictive model for stroke risk stratification in ICAS patients.
Methods
Design
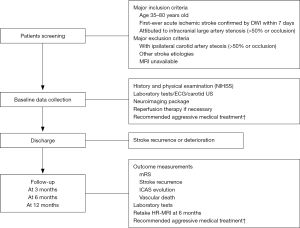
This is a multicenter, prospective, cohort study following participants for 3, 6 and 12 months from stroke onset. An overview of the schedule of this study is shown in Figure 1. This study was approved by the institutional review board at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (JS-1699, September 25, 2018) and local participating centers’ ethics board.
Study population
Eligibility criteria are: (I) age between 35–80 years old; (II) first-ever stroke confirmed by diffusion-weighted imaging (DWI) attributed to intracranial artery stenosis (>50% or occlusion) within 7 days after onset; (III) with stable vital signs. Patients who have underwent thrombolytic or thrombectomy therapy are allowed to enroll in this study. Patients who meet these criteria are informed the study and asked to sign the consent forms for enrollment.
Exclusion criteria include: (I) with >50% ipsilateral carotid artery stenosis, cardiac embolism and any other stroke etiologies such as vasculitis, dissection or other causes; (II) with absolute or relative contradiction to MRI including but not confined to metal in the body and claustrophobia; (III) cannot comply with MRI exam; (IV) decline the consent.
Clinical data collection
The following clinical parameters are collected during hospitalization: (I) demographic information; (II) risk factors such as hypertension, diabetic mellitus, hyperlipemia, smoking etc.; (III) other medical comorbidities or medical history; (IV) national institutional health stroke scale (NIHSS) score; (V) medications or other therapies. Routine laboratory examinations as well as electrocardiogram (ECG), echocardiography and carotid artery ultrasound (US) are also of necessity. The laboratory items are required to monitor side effects of treatments. During follow-up period, stroke recurrence defined by a focal neurological defect confirmed by new ischemic lesions on DWI and vascular death events should be recorded. The modified Rankin score (mRS) grading 0–6 are used to assess functional outcome in clinics or by phone.
Imaging protocol
Each patient will undergo a novel stroke imaging package including conventional cranial MRI (T1-weighted imaging, T2-weighted imaging, T2-weighted fluid attenuation inversion recovery imaging, DWI), three-dimensional time-of-flight (3D TOF) MRA, susceptibility-weighted imaging (SWI) or T2*-weighted imaging and 3D T1-weighted HR-MRI. Arterial spin labeling MRI, two-dimensional (2D) T2-weighted HR-MRI of intracranial arteries (bilateral middle cerebral arteries and basilar artery) and contrast-enhanced 3D T1-weighted HR-MRI are optional if available. The requirements of slice thickness are 5–6 mm for all conventional MR sequences and 1.5–3 mm for SWI at different units. The rest parameters like repetition time (TR), echo time (TE) or others are not requested and can be adjusted in order to obtain high imaging quality. The HR-MRI sequences should be performed on 3T MRI. The basic parameters of 3D T1-weighted HR-MRI in different MRI set are equal as following: variable flip angle 3D fast spin echo sequence (CUBE on GE, SPACE on SIEMENSE, VISTA on PHILIPS), TR: 500–900 ms, TE: <22 ms, acquired minimal voxel resolution: 0.8 mm × 0.8 mm × 0.8 mm [zero-filled Fourier transform (ZIP 512, ZIP2) is used to reduce pixel size and the final displaying resolution is 0.4 mm × 0.4 mm × 0.4 mm], scan range: >480 mm, included the Willis circle, M1, M2, and M3 segments of middle cerebral artery, vertebral artery, and basal artery. This imaging package with a total scan time of approximately 25–35 mins had been proved its feasibility and strengths in an another multicenter cohort study (unpublished data).
Patients will repeat HR-MRI imaging on the same machine at 6 months after the enrollment to ensure identity and comparability. Conventional cranial MRI is required if a new stroke occurs during follow-up period.
Treatments
Patients will be recommended to take aggressive medical treatments including dual antiplatelet [aspirin (100 mg daily) and clopidogrel (75 mg daily) ] for 3 months and rosuvastatin (20 mg daily) for at least 6 months as well as traditional risk factors managements including blood pressure control (targeting systolic blood pressure ≤140 mmHg); lipid lowering (targeting low-density lipoprotein cholesterol level <70 mg/dL and <50% of the baseline level); blood glucose level control; smoking cessation; weight management and physical activity according to the guidelines (14). If patients are unable to tolerate this standard therapy, local clinicians can adjust the treatment, record reasons and keep them in this cohort.
Outcomes
The primary outcome measurements are 90-day poor functional outcome defined as mRS >2 [(0) indicates no symptoms, (6) indicates death] and stroke recurrence during 12-month follow-up. In the meantime, the primary imaging outcome measurements are the evolution of ICAS including plaques and thrombus at 6 months. The second primary outcome is death events due to vascular etiologies during 12-month follow-up.
Data management and interpretation
Each participating center will collect clinical data and complete the pre-designed electronic case report form (eCRF) on a commercial internet database (http://ecrf.linklab.com).
All image data will be delivered to and stored in the core labs (Peking Union Medical College Hospital) for analysis. The images will be interpreted by a radiologist and a neurologist who blinded to patients’ clinical information independently using commercial software (OsiriX MD, v.9.02). The differences between the two observers will be solved by consensus. The characteristics of ICAS including degree of stenosis, artery remodeling, artery geometric features, and distribution, number, length, volume of plaques as well as components including intraplaque hemorrhage, lipid core or fibrous caps, thrombus and other surrounding items like deep tiny flow voids as shown by HR-MRI at baseline will be carefully measured and analyzed. What’s more, the dynamic changes of these pathological lesions under aggressive treatment will be observed. Other parameters like cerebral small vessel lesions, infarction pattern and volume will be recorded.
Sample size and statistical analysis
This is a cohort study with a primary aim to describe the imaging features and outcomes of patients with symptomatic ICAS patients under aggressive medical treatment. Therefore, we calculate the sample size for the logistic analysis for predicting stroke recurrence. Based on the criteria of at least 10 events per variable in the logistic regression model (15), we will need 50–80 events of stroke recurrence according to the analysis plan which we will have 5 to 8 variates in the logistic regression model. Assuming a recurrence rate of ischemic stroke for patients with symptomatic ICAS patients under modern medical treatment was 13% within 12 months based on previous studies (6,16), the sample size was approximately 385 to 615. With 400 participants with follow-up, we will be able to detect an odds ratio corresponding to a correlation of 1.7 or higher (power 88.5% at significant level 5%). Experience from our unpublished previous study called stroke imaging package study (SIPS), 80% of eligible subjects would be included finally and maximum up to 10% of participants would be lost to follow up during 12-month period. Hence the final sample size for screening was estimated at least 550 in total.
Demographics and participant characteristics will be presented using descriptive statistical analyses. Continuous data will be described with mean (standard deviation) or median (interquartile range) and categorical data will be percentile. Comparison of two data will be conducted with t-test, Mann-Whitney U test or Wilcoxon ranks sum test for continuous variables as appropriate and chi-square test or Fisher exact test for categorical variables. The associations between baseline characteristics, specific imaging markers and outcome events will be assessed by multivariable logistic regression. Stratified analysis and interaction analysis will be performed if necessary.
Results
Enrollment began in November 2018 and 96 patients have been enrolled at the end of September 2019. The enrollment and follow-up are currently ongoing, and interim analysis is expected in January 2020. The final results of this study will be presented at scientific meeting and published at peer-reviewed medical journals.
Discussion
ICAS is a major cause which accounts for a large amount of acute ischemic stroke patients with high disability and recurrence rate. The central consideration for effective treatment and prevention is to recognize, stabilize and even reverse vulnerability of ICAS. Vulnerable lesions are defined as lesions that are at high risk to thrombosis accompanied by clinical symptoms (2). In recent years, the capacity of HR-MRI in evaluating plaque vulnerability by quantitively measuring vessel wall and intracranial plaques have been explored (11,12,17). However, there is still lacking of unified standard of vulnerable intracranial lesions due to different results from small sample size researches and few longitudinal assessments.
SIPS-ICAS is a large cohort study which aims to provide a comprehensive and detailed evaluation of ICAS based on a novel stroke imaging package including HR-MRI. Firstly, it has the potential to identify distinct imaging features of high-risk lesions by comparing between patients with or without stroke recurrence, favorable or unfavorable function outcomes, symptomatic or asymptomatic atherosclerosis [approximately twenty percent of symptomatic ICAS patients have asymptomatic plaques at same time based on evidence from warfarin-aspirin symptomatic intracranial disease (WASID) trial investigators study] (18). What’s more, the dynamic changes like regression or progression of plaques shown by HR-MRI can fulfill the gap between effects of aggressive medical treatment and underlying mechanisms and lead to new understandings of pathophysiology of ICAS. Finally, a key point of this study is to show the link between imaging biomarkers and clinical events. Therefore, establishing a reliable predictive model beyond the degree of stenosis is anticipated.
The prominent limitation of the study is the lack of histological evidence to validate HR-MRI findings particularly for plaque components, although several intracranial MRI-histology comparative researches have shown the results consistent with ECAS findings (2).
Conclusions
All in all, SIPS-ICAS will pave the way for an overall understanding of ICAS and better clinical management. In addition, it will further demonstrate the practicability and value of this novel stroke imaging package and lay foundation to its widely clinical use.
Acknowledgments
Funding: This work was supported by the Ministry of Science and Technology of the People’s Republic of China (grant numbers 2017YFC1307900, 2017YFC1307902).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (JS-1699, September 25, 2018) and local participating centers’ ethics board. Written informed consents were obtained from the patients or their legal relatives.
References
- Kim JS, Bonovich D. Research on intracranial atherosclerosis from the East and west: why are the results different? J Stroke 2014;16:105-13. [Crossref] [PubMed]
- Yang WJ, Wong KS, Chen XY. Intracranial atherosclerosis: from microscopy to high-resolution magnetic resonance imaging. J Stroke 2017;19:249-60. [Crossref] [PubMed]
- Burke AP, Kolodgie FD, Farb A, et al. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002;105:297-303. [Crossref] [PubMed]
- Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012;71:195-8. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003. [Crossref] [PubMed]
- Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240-8. [Crossref] [PubMed]
- Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke 2014;45:645-51. [Crossref] [PubMed]
- Chen XY, Wong KS, Lam WW, et al. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis 2008;25:74-80. [Crossref] [PubMed]
- Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008;39:1142-7. [Crossref] [PubMed]
- Li ML, Xu WH, Song L, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis 2009;204:447-52. [Crossref] [PubMed]
- Li ML, Xu YY, Hou B, et al. High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence. Eur J Radiol 2016;85:803-7. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke 2011;42:2957-9. [Crossref] [PubMed]
- Wang Y, Liu M, Pu C. 2014 Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke 2017;12:302-20. [Crossref] [PubMed]
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Leung TW, Wang L, Soo YO, et al. Evolution of intracranial atherosclerotic disease under modern medical therapy. Ann Neurol 2015;77:478-86. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S. Intracranial plaque regression after intensive medical treatments: a high-resolution MRI observation. Ann Transl Med 2014;2:82. [PubMed]
- Nahab F, Cotsonis G, Lynn M, et al. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke 2008;39:1039-41. [Crossref] [PubMed]


