
Serum amyloid A1 as a biomarker for radiation dose estimation and lethality prediction in irradiated mouse
Introduction
Ionizing radiation (IR) due to industrial accidents, terrorist attacks or nuclear power plant accident, can severely damage physiological functions within hours to weeks even years depending on dose and dose rate, as well as producing long-term health problems among survivors (1-3). The degree of radiation damage is closely related to the dose of radiation. The use of radiation dose estimation techniques for a rapid classification of and dose estimation in the wounded is a key component of medical rescue. Furthermore, fast and reliable biomarkers are needed to distinguish whether individuals were exposed or not to radiation, to assess the radiation dose and, more importantly, predict the severity of the radiation damage.
There are several techniques that have been used to estimate the radiation dose after a radiation accident (4). Lymphocyte reduction kinetics analysis can be performed outside the laboratory, but requires multiple measurements within 48 hours after exposure (5,6). γ-H2AX foci analysis has been widely recognized as a reliable and sensitive radiation-induced DNA double-strand break marker, which must be performed in the laboratory, and the ideal time frame is just 1 to 3 hours after exposure (7-10). As the “gold standard” for biological dose estimation, chromosomal aberration analysis is characterized by a high accuracy and specificity. Nevertheless, it requires a long processing time and it is of high technical difficulty (11,12).
Serum amyloid A1 (SAA1) is an acute-phase protein, which can increase from 10 to 100 times due to local or mild inflammation to severe inflammation, respectively (13-15). The major site of SAA1 synthesis is the liver, and is principally induced by exogenous toll-like receptors ligands such as lipopolysaccharide (LPS) and endogenous cytokines such as interleukin 1 beta (IL-1β), interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) (15). Indeed, extrahepatic SAA1 expression was also found in human tissues such as breast, colon, oesophagus, kidney, large intestine, pituitary gland and spleen (15). The SAA1 protein encoded by the SAA1 gene is highly similar in both human and mouse, and plays a role in high-density lipoprotein remodelling, lipid metabolism, anti-bacterial infection, immune regulation, and tumour pathology (15,16).
Acute inflammatory response can be caused by radiation in the early stage (17,18), and radiation-induced multiple organ dysfunction caused by a systemic inflammatory response is considered as the main cause of death in the late stage of radiation (19). Therefore, we wondered whether SAA1 could be increased by radiation in the early stage and stimulated by radiation-induced systemic inflammatory response in the late stage of radiation. To test this hypothesis, a mouse irradiation model was established, and the change in SAA1 expression was evaluated in response to total and partial body irradiation in the early stage. In addition, its role in predicting long-term impact and prognosis was also analysed. Subsequently, serum SAA1 concentration was measured before and after radiotherapy in nasopharyngeal carcinoma patients to estimate the feasibility of SAA1 as a biomarker in human radiation exposure.
Methods
Animals
C57BL/6J female mice (6–8 weeks old) were obtained from the Beijing Vital River Laboratory Animal Technology and raised at the Academy of Military Medical Sciences (Beijing, China). Mice were housed in unified cages under specific pathogen-free conditions with a controlled temperature, humidity, under a 12/12 h light/dark cycle, and were kept for at least one week and weighing 19–21 g before the experimental treatment. Animal care and handling were performed in accordance with the ‘Guide for the Care and Use of Laboratory Animal of AMMS in China’ and all animal experiments were approved by the Animal Care and Use Committee of Beijing Institute of Radiation Medicine (Beijing, China).
Total body irradiation (TBI)
As regard the time and dose response study, mice were placed in plexiglass transparent boxes to prevent movements and exposed to a single dose of 1, 2, 4, 8 and 12 Gy or sham irradiation using 60Co source γ-ray at a dose rate of 85.08 cGy/min. At different time points (0.25, 0.5, 1, 2, 3, 5 and 7 days), 20 µL blood were collected from the tail vein for blood counts and it was also collected from the orbital plexus. Mice were then sacrificed by decapitation and a sample of liver, lung, thymus, spleen, small intestine, and bone marrow was collected from control and 8 Gy irradiated samples at the time point of 0.125, 0.5, 1, 2, 3, 5 and 7 days and stored at −80 °C. Serum was isolated by centrifugation at 5,000 rpm/min for 5 min at 4 °C and stored at −80 °C. Another group of mice under the same radiation conditions as described above was used to collect blood from the tail vein and weighed at 1, 3, 7, 11, 15, 20, 25, and 30 days after radiation to observe the survival until day 30.
As regard the study of dynamic SAA1 concentration, mice were randomly numbered by earrings with unique numbers for each one and left for 5 days to restore the normal condition. Mice of the 10 Gy group were placed in plexiglass transparent boxes to prevent movements and exposed to a single dose of 10 Gy irradiation using 60Co source γ-ray at a dose rate of 73.02 cGy/min. Approximately 40 µL blood were collected from the tail vein of each mouse at −4, 1, 3, 5, 7 days after irradiation, and the survival of each mouse was monitored until day 30. Serum was isolated by centrifugation at 5,000 rpm/min for 5 min at 4 °C and stored at −80 °C.
Partial body irradiation (PBI)
Mice were anesthetized with and intraperitoneal injection of 10 mg/kg pentobarbital sodium and then placed on plexiglass boxes at unified position. Lead bricks were used to shield mice in order to expose only the desired body parts. At day 0.5 after 8 Gy irradiation using 60Co source γ-ray at a dose rate of 85.08 cGy/min, 20 µL blood were collected from the tail vein for blood counts and then it was also collected from the orbital plexus. Mice were then sacrificed by decapitation and liver was removed and stored at −80 °C. Serum was isolated by centrifugation at 5,000 rpm/min for 5 min at 4 °C and stored at −80 °C.
Amifostine treatment
Mice were randomly numbered by earrings with unique numbers for each one and left for 5 days to restore the normal condition. Amifostine was intraperitoneally injected to the mice of the 10 Gy + Amifostine group at a dose of 150 mg/kg at 0.5 hour before 10 Gy irradiation, and injected to the mice of Amifostine group at a dose of 150 mg/kg at 0.5 hour before sham irradiation. Approximately 40 µL blood were collected from the tail vein of each mouse at −4, 1, 3, 5, 7 days after 10 Gy irradiation or sham-irradiation, and the survival of each mouse was monitored until day 30. Serum was isolated by centrifugation at 5,000 rpm/min for 5 min at 4 °C and stored at −80 °C.
ELISA measurement
Serum proteins such as SAA1 and procalcitonin (PCT) were measured using enzyme-linked immunosorbent assay (ELISA) kits (Mouse SAA1: EK1190, Boster, Wuhan, China; Human SAA1: EK1544, Boster, Wuhan, China; PCT: E10371m, CUSABIO, Wuhan, China) according to the manufacturer’s instructions. SAA1 and PCT concentrations in serum samples were determined via the calibration curve using standard proteins.
LPS assay
Tachypleus Amebocyte Lysate (EC80545, Bioendo, Xiamen, China) was used to detect LPS in the serum. A total of 10 µL serum was diluted in 90 µL sample treatment solution in a pyrogen-free test tube that was subsequently placed in a water bath at 70 °C for 10 min followed by an ice bath for 3 min. The protocol was performed according to the manufacturer’s instructions. The LPS concentration in serum samples was determined via the calibration curve using standard endotoxin.
RNA isolation and quantitative PCR analysis
An appropriate amount of tissue was cut into the EP tube, grinded in 200 µL TRIzol (15596018, Invitrogen, Carlsbad, CA, USA), and then 1 ml TRIzol was added. Total RNA was isolated according to TRIzol manufacturer’s instructions. The cDNA was synthesized using PrimeScript RT reagent kit (RR047A, Takara, Shiga, Japan) according to the manufacturer’s instructions. Quantitative PCR was performed using iTaq Universal SYBR Green Supermix (172-5125, BioRad, Rachmond, CA, USA) on a BioRad CFX96 using primers listed in Table S1.

Full table
DNA isolation and quantitative PCR analysis
An appropriate amount of liver was cut and placed into the EP tube containing 200 µL saline. Total DNA was isolated using TIANamp Genomic DNA Kit (DP304, TIANGEN, Beijing, China) according to the manufacturer’s instructions. Quantitative PCR was performed using iTaq Universal SYBR Green Supermix (172-5125, BioRad, Rachmond, CA, USA) on a BioRad CFX96 using 16S rRNA gene targeted primers listed in Table S1.
Patients and serum samples
The serum was collected from 17 patients with nasopharyngeal carcinoma who signed a written informed consent at the Nanfang Hospital, China. All specimens derived from a Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (LC2016 PY015), which could be found at https://www.clinicaltrials.gov/, and the research protocol was approved by the ethics committee/institution (No. NFEC-2018-013). Well-collimated photon beams from a 6 MV linear accelerator were used to encompass the nasopharynx and its adjacent regions, such as the posterior nasal fossae, parts of the paranasal sinuses, parapharyngeal spaces, and the skull base. The total dose to the target volume was 70 Gy to the nasopharynx and 63 Gy to the neck. Blood samples were collected 3–7 days before and 1–4 days after radiation therapy. Then, serum was isolated by centrifugation at 3,000 rpm/min for 10 min at 25 °C and stored at −80 °C.
Statistical analysis
Statistical analysis was performed using IBM SPSS 21 (Cary, NC, USA). Data with a normal distribution were analysed by the Student’s t-test to evaluate significant difference between two groups, otherwise Nonparametric Tests was used. For the statistical analysis of serum SAA1 levels as a predictor of the radiation dose in TBI, Multiple Linear Regression Analysis was performed. Bivariate correlation analysis among SAA1 and LPS or 16S rRNA on 8 Gy irradiation mice, and SAA1 and PCT on 12 Gy irradiation mice were performed using non-parametric Spearman rank correlation, as data were not normally distributed. Predictive cut-off values were identified from the receiver operating characteristic (ROC) curve. Survival distribution was determined by log-rank test. Mann-Whitney U test was used for comparison of SAA1 concentration after radiotherapy in patients with nasopharyngeal carcinoma. Results were expressed as mean ± standard deviation (SD). A P value <0.05 was considered statistically significant.
Results
TBI increased serum SAA1 levels in C57BL/6J mice
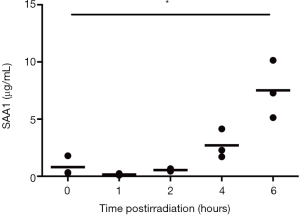
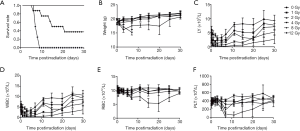
To evaluate the effect of total body irradiation at different doses and time, serum SAA1 levels in C57BL/6J mice receiving TBI at 1, 2, 4, 8 and 12 Gy after 0.25, 0.5, 1, 2, 3, 5 and 7 days were detected by ELISA. As shown in Figure S1, SAA1 concentration slightly increased from 4 hours and increased significantly at 6 hours after 8 Gy irradiation. Figure 1 shows the SAA1 dose and time-dependent change and P values associated with two-tailed Student’s t-test are presented in Table S2. As shown in Figure 1A, a moderate increase in serum SAA1 levels was observed in the irradiated samples at all doses at the time point of 6 hours, reaching the peak at 12 hours in all the dose groups, then decreased until 3 days after exposure. Figure 1B,C,D,E,F,G,H show the SAA1 dose responses at 0.25, 0.5, 1, 2, 3, 5 and 7 days after irradiation, respectively. At day 5 after irradiation, a substantial increase in SAA1 was observed in 2 (60.66 and 113.26 µg/mL) of the 8 mice in the 8 Gy group, and 6 (109.62, 301.87, 365.43, 424.15, 332.16, and 284.66 µg/mL) of the 8 mice in the 12 Gy group. At day 7 after irradiation, SAA1 increase was observed in 4 (156.79, 106.12, 170.22, and 107.31 µg/mL) of the 7 mice (1 of 8 mice died at day 6 after irradiation) in the 8 Gy group, and the surviving 2 (234.23 and 114.54 µg/mL) in 8 mice of the 12 Gy group. The survival rate, weight, and complete blood count of the other group of mice with the same radiation conditions are shown in Figure S2. The number of mice showing a second SAA1 increase was approximately the same as the number of deaths in another group of mice under the same radiation conditions suggesting that the second SAA1 increase might represent a biomarker for death induced by radiation.

Full table
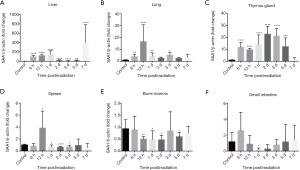
TBI induced expression of SAA1 mRNA in C57BL/6J mice tissues
To confirm the source of SAA1 due to irradiation, SAA1 mRNA expression in various tissues of C57BL/6J mice after TBI was measured. As shown in Figure 2, the highest SAA1 mRNA expression was in the liver, followed by thymus, lung and spleen, although no significant change was observed in the bone marrow (Figure 2E) and small intestine (Figure 2F). Figure 2A shows SAA1 mRNA fold change increase in liver from 6 hours, with a peak at day 1 of 147.57±76.65, while modestly increased from day 2 to 5, reaching a higher peak at day 7 of 394.53±389.31. Figure 2B shows that SAA1 mRNA increased in the lung at the time point of 0.125, 0.5, 1 and 3 days, and the highest expression of 16.56-fold ±14.14 was reached on day 0.5 post-irradiation. Figure 2C shows that SAA1 mRNA expression in the thymus was significantly increased at the time point of 0.125, 0.5, 1, 2, 3 and 5 days. Figure 2D shows SAA1 mRNA expression in spleen, with a peak of 3.83±2.84 at 12 hours, while marginally increased at 1, 2 and 3 days.
SAA1 increase in serum was not due to systemic infection
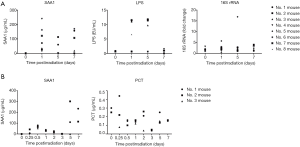
Since systemic infection is one of the symptoms of acute radiation sickness after TBI (20), we wondered whether the increase of SAA1 was induced by systemic infection. As the direct factor of bacterial infection, LPS is the predominant endotoxin in gram negative bacteria and 16S rRNA is a DNA sequence corresponding to the rRNA encoded by bacteria and is present in the genome of all bacteria. Bacterial translocation was determined as the bacterial load in the liver tissue and can be quantified by Quantitative PCR using the 16S rRNA gene consensus sequence (21). Therefore, serum LPS in and liver 16S rRNA were measured in the same animals, on 31 mice in total and on days 0, 1, 5 and 7 (7 mice on days 7 and 8 per group on days 0, 1 and 5) after exposure to 8 Gy TBI (Figure 3A). No one-to-one correspondence was observed between the indicators in the same mouse. Using the parameters of 7 mice after 7 days of irradiation at 8 Gy as an example, the SAA1 concentration in the 7 mice was 156.79, 0.12, 18.92, 0.51, 106.12, 170.22, and 107.31 µg/mL, the LPS level was 0.97, 0.03, 0.18, 1.56, 0.03, 0.06, and 0.98 EU/mL, and the fold change of 16S rRNA in the liver was 1.14, 4.03, 1.73, 1.28, 2.39, 3.44, and 3.39, respectively. Besides bacterial infection indicators such as LPS and 16S rRNA, PCT as a marker of bacterial infection (22,23) was measured in the same animals on 24 mice on days 0, 0.125, 0.5, 1, 2, 3, 5 and 7 (2 mice on days 7 and 3 per group on days 0, 0.125, 0.5, 1, 2, 3 and 5) after exposure to 12 Gy TBI (Figure 3B). For example, the SAA1 concentration on day 1 and 5 was respectively 38.26, 28.40, 40.11 µg/mL and 109.62, 301.87, 0.25 µg/mL, while the PCT concentration on day 1 and 5 was 0.14, 0.16, 0.12 ng/mL and 0.25, 0.13, 0.15 ng/mL. Bivariate correlation analysis among SAA1 and LPS or 16S rRNA on 8 Gy irradiation mice, and SAA1 and PCT on 12 Gy irradiation mice were performed, resulting not significant between the infection parameters and SAA1 expression.
Role of serum SAA1 level in the radiation dose estimation in mice after TBI
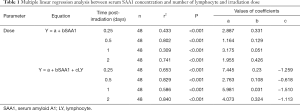
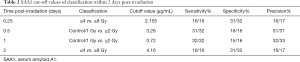
To explore whether serum SAA1 levels could be used as predictor of the radiation dose in TBI mice, a multiple linear regression analysis was conducted, with SAA1 concentration and total lymphocyte count of peripheral blood (Figure S2) as independent factors and radiation dose as dependent factor, at the time point of 0.125, 0.5, 1, 2 days. Results of the regression analysis indicated that the radiation dose in TBI mice could be estimated by measuring serum SAA1 concentrations alone within 2 days after exposure, especially on day 0.5 post-irradiation when the correlation coefficient was r2=0.802 (n=48, P<0.001) (Table 1). Furthermore, combining SAA1 concentration with total lymphocyte count in the peripheral blood together as independent variables could result in a better effect of estimating the irradiation dose, especially on day 0.5 and 2 post-irradiation when the correlation coefficient was r2=0.829 (n=48, P<0.001) and r2=0.840 (n=48, P<0.001), respectively. Besides the dose of radiation, classification of patients according to the dose range was also important and required. The receiver operator curve (ROC) was used to identify the SAA1 µg/mL threshold within 2 days post-irradiation to establish sensitivity, specificity and precision (Table 2).
Full table
Full table
Increased SAA1 levels in mice after PBI
In radiation accidents, PBI is a more common situation than TBI (24). As shown above, liver is the main source of SAA1 after TBI, and the change in SAA1 is unknown under the condition of shielding of the liver. To explore the levels of SAA1 under shielding liver conditions, four PBI patterns were conducted by exposing to radiation the head and the upper half of the chest (PBI-1), shielding the head and the upper half of the chest (PBI-4), shielding the low-half abdomen and hind legs (PBI-2), and exposing to radiation the low-half abdomen and hind legs (PBI-3). As can be seen in Figure 4, the only difference between PBI-1 and PBI-2 was that in the PBI-1 the liver was shielded, while in PBI-2 it was not; similarly, in the PBI-3 the liver was shielded, while in PBI-4 it was not. SAA1 protein concentrations in serum and SAA1 mRNA fold change in liver were measured at 12 hours after 8 Gy irradiation. Compared with the control group, SAA1 protein concentrations in serum and SAA1 mRNA fold change in liver exposure group (PBI-2 and PBI-4) were higher than liver shielding group (PBI-1 and PBI-3). Furthermore, although the liver, as the major tissue producing SAA1 as reported before, was shielded, no matter if SAA1 protein in serum or SAA1 mRNA in liver was considered in PBI-1 and PBI-3 group, because they were all increased than the control. To verify whether the cut-off value of TBI on day 0.5 post-irradiation was suitable for PBI, the SAA1 concentration of 3.26 µg/mL was selected to distinguish control group and PBI group (PBI-1, PBI-2, PBI-3, PBI-4), then the sensitivity of 22/24, specificity of 24/24 and precision of 24/24 were obtained. Therefore, these results suggested that the cut-off value of TBI at day 0.5 after radiation was also applicable to local irradiation. Then, to explore whether SAA1 could be a biomarker for the irradiation area of PBI in mice, the receiver operator curve (ROC) was used to distinguish the group small area partial irradiation without liver (PBI-1 and PBI-3) from the group large area partial irradiation including liver (PBI-2 and PBI-4), obtaining a cut off value of 11.46 µg/mL, sensitivity of 12/12, specificity of 11/12 and precision of 12/13.

Correspondence between SAA1 second increase and subsequent lethality in mice after irradiation
As shown in Figure 1H, a second increase of SAA1 occurred in some mice from day 5 to 7 after 8 Gy and 12 Gy irradiation. Coincidentally, the number of mice with second SAA1 increase was approximately the same as the number of deaths in mice of model group. To evaluate whether the second SAA1 increase could be a useful indicator of death induced by radiation, dynamic serum samples were collected from 20 mice of the 10 Gy group at 5 times (4 days prior to exposure and 1, 3, 5, 7 days after exposure). Dynamic SAA1 concentration and corresponding survival time after 10 Gy irradiation of each mouse are shown in Table 3, in which each row represents the SAA1 concentration change for continuous blood sample from a single animal and the corresponding survival time. A total of 92 healthy mice were used to calculate mean and standard deviation of SAA1 concentrations (Table S3), using the cut-off value of 1.15 µg/mL (mean + 2SD) to predict subsequent lethality within 30 days after irradiation. On day 5, SAA1 concentration in 8 of the 17 dead mice and in 1 of the 3 live mice was above 1.15 µg/mL, the sensitivity was 8/17, specificity was 2/3, and precision was 8/9. In addition, on day 7 SAA1 concentration in 13 of the 16 dead mice and in 1 of the 3 live mice was above 1.15 µg/mL, the sensitivity was 13/16, specificity was 2/3, and precision was 13/14.

Full table

Full table
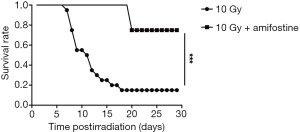
After determining the correlation between SAA1 second increase and subsequent lethality in mice after irradiation, amifostine [a cytoprotective agent (25)] effect was assessed on SAA1 second increase to evaluate the role of this second increase in mice after radiation. As shown in Figure 5, mice exposed to 10 Gy started to die at day 7 and only 3 of 20 mice survived until day 18 in the 10 Gy group, while in the 10 Gy + Amifostine group only 2 of 8 mice receiving amifostine before radiation exposure died on day 20 (P=0.001). Under the premise that the amifostine treatment had no effect on SAA1 expression (Table S4), no significant difference in SAA1 was observed between the 10 Gy group and 10 Gy + Amifostine group before irradiation at day −4, 1, 3 and 5. Surprisingly, treatment with amifostine before 10 Gy irradiation could inhibit the second increase of SAA1 on day 7 (P=0.004), and only 2 of 8 mice in the 10 Gy + Amifostine group died at 20 day after radiation, which also indicate that secondary SAA1 increase could be considered as an useful indicator of death induced by radiation.
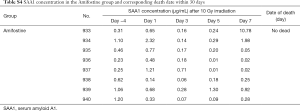
Full table
Increased serum SAA1 levels in nasopharyngeal carcinoma patients after radiotherapy
Since it was proved that SAA1 could be used as a radiation biomarker in a mouse model, we wondered whether SAA1 could be used as a biomarker also in humans subjected to radiotherapy. Since from the ethical and moral aspects a human irradiation model is not allowed, serum SAA1 changes were evaluated after radiotherapy in patients with nasopharyngeal carcinoma. Table S5 shows the clinical parameters and corresponding concentration of SAA1 in all 17 nasopharyngeal carcinoma patients considered in the present study. As can be seen in Figure 6A, the serum SAA1 concentration in each patient was increased compared with that before irradiation, and the median value of concentration after radiotherapy (44.63 µg/mL) was considerably higher than the pre-therapeutic concentration (0.54 µg/mL). Figure 6B shows the ROC curve of SAA1 as a biomarker in discriminating radiation exposure of patients with nasopharyngeal carcinoma, the area under the curve (AUC) was 0.941±0.05, with a cut-off value of 9.34 µg/mL and corresponding sensitivity of 18/18, specificity of 16/18, and precision of 18/20.
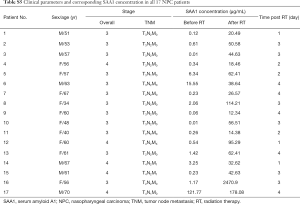
Full table
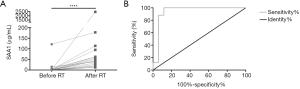
Discussion
Lymphocyte depletion and analysis of chromosomal aberrations are the main biomarkers used in the current diagnostic screening to estimate the dose of accidental radiation exposure. However, the former is hardly allowing to draw definite conclusions and the latter is time-consuming (26,27). The current progress in protein identification techniques using proteomics allows to distinguish and identify all proteins in the mixed protein sample (28). In particular, with the development of protein detection technologies, which are fast and convenient, such as enzyme linked immunosorbent assay and colloidal gold immunochromatography assay, protein biomarkers have the potential to lead a new direction in the radiological diagnostic technology. Several studies are available regarding SAA1 increase, even as a biomarker, after radiation exposure. Dose and time dependent concentration of plasma SAA1 in C57BL/6J strain mice during 1–7 day after radiation exposure ≤6 Gy is increased only during the first two days, exhibiting maximum changes at day 1 (29). Furthermore, a study using female C57BL/6J mice receiving 1–8 Gy dose X-ray source indicated that using SAA1 levels at 24 h as a dose prediction model could successfully differentiated TBI mice into dose received cohorts of control/1 Gy and ≥2 Gy groups with a high degree of accuracy in a blinded study (30). In the event of a radiological incident, large-scale population screening is required to classify exposed individuals, and the evaluation of the subsequent exposure level is an important preliminary screening for emergency victims of mass casualties (3). Depending on the exposure dose, the treatment may be minimal, involving only supportive care, or extensive, requiring intervention, such as stem cell transplantation (31,32). Hence accurate dosimetry that could estimate exposure dose in individual patients is required for discreet deployment of medical resources and care (33). In our study, the post-irradiation time was set at 6 h and the irradiation dose used was set at 12 Gy. A moderate increase of SAA1 was detected already at 6 hours, and the concentration reached a peak at 12 hours in all dose groups, which was different from previous studies. Moreover, the slight increase of SAA1 within 2 days after irradiation was maintained. Therefore, multiple linear regression analysis between serum SAA1 concentration and irradiation dose was performed, and the results demonstrated that serum SAA1 could predict the exposure dose within 2 days post-irradiation, especially at 12 hours. Furthermore, lymphocyte depletion kinetics is one of the current diagnostic screening to estimate the dose of radiation exposure, combining together SAA1 concentration and total lymphocyte count of peripheral blood as independent variables allow a better estimation of the irradiation dose. Therefore, the acute increase of SAA1 within 2 days after radiation exposures might be used as a protein biomarker to estimate the radiation dose.
A key issue that was successfully identified in this work was the correlation of serum second increase of SAA1 with the subsequent lethality in irradiated mice. This is a crucial aspect because under the premise of estimating the dose of radiation, it is still a challenge the identification of seriously damaged individuals who were subjected to the same radiation dose (34). Under the same dose, the degree of individual injury can be very different due to individual differences, thus, directly assessing the degree of radiation damage is exactly the ultimate goal. In our study, SAA1 could recognize severely injured individuals and predict subsequent death by the second increase to 1.15 µg/mL from day 5 to 7 after 10 Gy irradiation. As the sensitivity of 8/17 on day 5 was lower than the sensitivity of 13/16 on day 7, we hypothesized that the SAA1 concentration at day 7 was more sensitive and useful for predicting subsequent lethality. However, due to individual differences between mice, we believe that the secondary increase of SAA1 was not limited to the concentration level at day 5 or 7 after radiation, but more like a tendency to rise rapidly after a fall at any time after irradiation. Because except for the mice No. 434 which died lately on the 18th day and No. 610 which died on the 7th day, so that blood cannot be collected, among all 15 dead mice, the SAA1 concentration on day 7 were higher than that on day 5. Among the three survived mice, SAA1 concentration in No. 607 increased a second time on day 5 while decreased to 0.68 µg/mL on day 7 and surprisingly survived, SAA1 concentration of No. 430 did not increased after day 3 and remained low. Although SAA1 concentration of No. 611 increased on day 7 to 8.62 µg/mL, we believe that the reason for survival could be that SAA1 decreased after day 7 or because of individual differences between mice. Therefore, in clinical applications, continuous monitoring of serum SAA1 concentration in patients would be of utmost importance for assessing prognosis. In addition, treatment with amifostine, a radioprotective agent which can reduce radiation damage (25), administered before 10 Gy of irradiation could inhibit the second increase of SAA1 although the first increase was still present. This result further confirmed that SAA1 second increase could be a useful indicator of survival after radiation. The mechanism of SAA1 increase during inflammatory response is known. It is synthesized by hepatocytes and driven by proinflammatory cytokines including IL-1β, IL-6 and TNF-α (15,35). Nuclear transcription factor-κB (NF-κB) is known as a major transcription factor stimulated by ionizing radiation, which induces inflammatory cytokines following irradiation, including IL-1β, IL-6 and TNF-α (36). Although there is currently no research to explore the mechanism of SAA1 increase after radiation, we hypothesized that its increase should be related to NF-κB activation based on existing studies. In our study, we confirmed that the increase of SAA1 after exposure was mostly related to the liver, since the first or the second SAA1 increase are related to liver mRNA levels. On the other hand, high SAA1 mRNA expression was also observed in immune organs such as the thymus and spleen. Therefore, we have enough reason to believe that the increase in SAA1 caused by radiation was related to immunomodulation. The late progression and symptoms of acute radiation sickness are very similar to classic infection (6,17), in which the concentration of SAA1 can dramatically increase (37). LPS, as an exogenous toll-like receptor ligands inducing SAA1, and PCT, are both biomarkers of bacteraemia (38-40). 16S rRNA gene, highly conserved in bacteria, was reported as a standard method to identify and classify prokaryotes (41,42). Thus, SAA1, PCT and LPS were measured in the same serum and 16S rRNA expression was evaluated in the liver of the same mouse, resulting in SAA1 increase not associated with infection. Therefore, non-infectious immune regulation could be the mechanism of SAA1 increase in radiation, and the specific reasons need to be further explored.
Furthermore, besides the mouse irradiation model, we also tested serum SAA1 concentration before and after radiotherapy in tumor patients to estimate the feasibility of SAA1 as a biomarker in human radiation exposure. Therefore, the following points were demonstrated in this work. Firstly, SAA1 is a feasible biomarker of radiation damage in mice, primates (43,44), or humans. Secondly, the threshold for diagnosing whether a patient with nasopharyngeal cancer is exposed to radiation is 9.34 µg/mL. Considering that SAA1 in a healthy person is low to an almost undetectable level and in nasopharyngeal carcinoma patients is less than 2-fold higher than that of normal people (45), it is credible that the threshold of predicting the exposure in practical applications could be lower than 9.34 µg/mL. Finally, since the location of radiotherapy in nasopharyngeal carcinoma is mainly near the nasopharynx and its adjacent regions, and the SAA1 in serum is mainly produced and released by the liver, this evidence demonstrated that in the process of radiation injury, a large amount of SAA1 could still be produced without directly irradiating the liver, which was also demonstrated in the mouse radiation model shown in Figure 4.
Conclusions
The change in SAA1 in response to TBI at different doses and time points was evaluated, confirming the role of SAA1 in the radiation dose estimation in the early stage of radiation damage in a mouse irradiation model. It was also demonstrated that the major site of SAA1 synthesis was the liver and the association between increased SAA1 and post-radiation infection was excluded. Subsequently, SAA1 second increase was successfully identified as a potential biomarker of predicting subsequent lethality of irradiated mouse. Finally, SAA1 resulted also a biomarker in human radiation exposure by detecting it before and after radiotherapy in nasopharyngeal carcinoma patients.
Acknowledgments
Funding: This study was supported by the Major Project: BWS18J008 and the Major Project: AWS14C014.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Animal care and handling were conduct in accordance with the ‘Guide for the Care and Use of Laboratory Animal of AMMS in China’ and all animal experiments were approved by the Animal Care and Use Committee of Beijing Institute of Radiation Medicine (Beijing, China). All experiments on human subjects were conducted in accordance with the Declaration of Helsinki. This study was approved by the Medical Ethical of Nanfang Hospital (No. NFEC-2018-013), and written informed consent was obtained from all the patients. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chin FK. Scenario of a dirty bomb in an urban environment and acute management of radiation poisoning and injuries. Singapore Med J 2007;48:950-7. [PubMed]
- Coeytaux K, Bey E, Christensen D, et al. Reported radiation overexposure accidents worldwide, 1980-2013: a systematic review. PLoS One 2015;10:e0118709. [Crossref] [PubMed]
- Pernot E, Hall J, Baatout S, et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat Res 2012;751:258-86. [Crossref] [PubMed]
- Marchetti F, Coleman MA, Jones IM, et al. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol 2006;82:605-39. [Crossref] [PubMed]
- Goans RE, Holloway EC, Berger ME, et al. Early dose assessment following severe radiation accidents. Health Phys 1997;72:513-8. [Crossref] [PubMed]
- Berger ME, Christensen DM, Lowry PC, et al. Medical management of radiation injuries: current approaches. Occup Med (Lond) 2006;56:162-72. [Crossref] [PubMed]
- Sandrine R-L, Tania M, Pascale V, et al. Quantification of gamma-H2AX foci in human lymphocytes: a method for biological dosimetry after ionizing radiation exposure. Radiat Res 2010;174:185-94. [Crossref] [PubMed]
- Ivashkevich A, Redon CE, Nakamura AJ, et al. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 2012;327:123-33. [Crossref] [PubMed]
- Xu Z, Yan Y, Xiao L, et al. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS One 2017;12:e0175977. [Crossref] [PubMed]
- Lu J, Tang M, Li H, et al. EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett 2016;380:191-200. [Crossref] [PubMed]
- Pinto MM, Santos NF, Amaral A. Current status of biodosimetry based on standard cytogenetic methods. Radiat Environ Biophys 2010;49:567-81. [Crossref] [PubMed]
- Sullivan JM, Prasanna PG, Grace MB, et al. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations. Health Phys 2013;105:540-54. [Crossref] [PubMed]
- Sung HJ, Ahn JM, Yoon YH, et al. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J Proteome Res 2011;10:1383-95. [Crossref] [PubMed]
- Connolly M, Marrelli A, Blades M, et al. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol 2010;184:6427-37. [Crossref] [PubMed]
- De Buck M, Gouwy M, Wang JM, et al. Structure and Expression of Different Serum Amyloid A (SAA) Variants and their Concentration-Dependent Functions During Host Insults. Curr Med Chem 2016;23:1725-55. [Crossref] [PubMed]
- Sun L, Ye RD. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016;583:48-57. [Crossref] [PubMed]
- Gordon LE, Ruml D, Hahne HJ, et al. Studies on susceptibility to infection following ionizing radiation. IV. The pathogenesis of the endogenous bacteremias in mice. J Exp Med 1955;102:413-24. [Crossref] [PubMed]
- Van der Meeren A, Monti P, Lebaron-Jacobs L, et al. Characterization of the acute inflammatory response after irradiation in mice and its regulation by interleukin 4 (Il4). Radiat Res 2001;155:858-65. [Crossref] [PubMed]
- Dainiak N, Gent RN, Carr Z, et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep 2011;5:183-201. [Crossref] [PubMed]
- Gluzman-Poltorak Z, Mendonca SR, Vainstein V, et al. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol 2014;7:31. [Crossref] [PubMed]
- Hillis DM, Moritz C, Porter CA, et al. Evidence for biased gene conversion in concerted evolution of ribosomal DNA. Science 1991;251:308-10. [Crossref] [PubMed]
- Procalcitonin Davies J. J Clin Pathol 2015;68:675-9. [Crossref] [PubMed]
- Nakamura A, Wada H, Ikejiri M, et al. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock 2009;31:586-91. [Crossref] [PubMed]
- Kaur A, Ten Have GAM, Hritzo B, et al. Morphological and functional impairment in the gut in a partial body irradiation minipig model of GI-ARS. Int J Radiat Biol 2018.1-52. [Epub ahead of print]. [Crossref] [PubMed]
- Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Semin Radiat Oncol 2003;13:62-72. [Crossref] [PubMed]
- Summaries for patients. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 2004;140:1037-51. [Crossref] [PubMed]
- Goans RE, Holloway EC, Berger ME, et al. Early dose assessment in criticality accidents. Health Phys 2001;81:446-9. [Crossref] [PubMed]
- Fenech M. Current status, new frontiers and challenges in radiation biodosimetry using cytogenetic, transcriptomic and proteomic technologies. Radiat Meas 2011;46:737-41. [Crossref]
- Kim D, Marchetti F, Chen Z, et al. Nanosensor dosimetry of mouse blood proteins after exposure to ionizing radiation. Sci Rep 2013;3:2234. [Crossref] [PubMed]
- Sproull M, Kramp T, Tandle A, et al. Serum Amyloid A as a Biomarker for Radiation Exposure. Radiat Res 2015;184:14-23. [Crossref] [PubMed]
- Slezak J, Kura B, Ravingerova T, et al. Mechanisms of cardiac radiation injury and potential preventive approaches. Can J Physiol Pharmacol 2015;93:737-53. [Crossref] [PubMed]
- Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol 2004;80:3-10. [Crossref] [PubMed]
- Anno GH, Baum SJ, Withers HR, et al. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5-30 Gy. Health Phys 1989;56:821-38. [Crossref] [PubMed]
- Kalman NS, Zhao SS, Anscher MS, et al. Current Status of Targeted Radioprotection and Radiation Injury Mitigation and Treatment Agents: A Critical Review of the Literature. Int J Radiat Oncol Biol Phys 2017;98:662-82. [Crossref] [PubMed]
- Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J 1998;334:489-503. [Crossref] [PubMed]
- Zhou D, Yu T, Chen G, et al. Effects of NF-kappaB1 (p50) targeted gene disruption on ionizing radiation-induced NF-kappaB activation and TNFalpha, IL-1alpha, IL-1beta and IL-6 mRNA expression in vivo. Int J Radiat Biol 2001;77:763-72. [Crossref] [PubMed]
- Ahmad S, Tejuja A, Newman KD, Zarychanski R, Seely AJ. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care 2009;13:232. [Crossref] [PubMed]
- Shenep JL, Flynn PM, Barrett FF, et al. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis 1988;157:565-8. [Crossref] [PubMed]
- Thomas-Rüddel DO, Poidinger B, Kott M, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Critical Care 2018;22:128. [Crossref] [PubMed]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010;14:R15. [Crossref] [PubMed]
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 1995;59:143-69. [PubMed]
- Greisen K, Loeffelholz M, Purohit A, et al. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol 1994;32:335-51. [PubMed]
- Ossetrova NI, Sandgren DJ, Blakely WF. C-reactive protein and serum amyloid A as early-phase and prognostic indicators of acute radiation exposure in nonhuman primate total-body irradiation model. Radiat Meas 2011;46:1019-24. [Crossref]
- Ossetrova NI, Sandgren DJ, Blakely WF. Protein biomarkers for enhancement of radiation dose and injury assessment in nonhuman primate total-body irradiation model. Radiat Prot Dosimetry 2014;159:61-76. [Crossref] [PubMed]
- Lung HL, Man OY, Yeung MC, et al. SAA1 polymorphisms are associated with variation in antiangiogenic and tumor-suppressive activities in nasopharyngeal carcinoma. Oncogene 2015;34:878-89. [Crossref] [PubMed]


