
Epiploic appendagitis: pathogenesis, clinical findings and imaging clues of a misdiagnosed mimicker
Introduction
Epiploic appendagitis is a rare cause of acute abdominal pain occurring predominantly in males in the fourth and fifth decade of life with an incidence of approximately 8.8 cases/106 population/year (1,2). Nevertheless, cases have been reported in children, even at the age of 5, as well as in the elderly population (3,4). Primary epiploic appendagitis (PEA) is the result of torsion of the appendage or thrombosis of the draining vein that cause ischemic necrosis and subsequent inflammation of the affected epiploic appendage (5-7).
PEA has been reported to occur more frequently in obese patients (8,9). Nugent et al., in a retrospective case control study involving patients with PEA and patients presenting with other causes of acute abdomen, reported that PEA subgroup had 60% greater abdominal adipose volume and 117% increased visceral adipose area (10). Other factors associated with PEA include intensive strenuous exercise and presence of hernia (8,9,11,12).
PEA is frequently misdiagnosed and commonly a mimicker of other serious causes of acute abdomen (13,14). Previously, it was considered a surgically treated disease and was most frequently diagnosed at surgery, but currently is treated conservatively with avoidance of unnecessary surgical interventions (1,7,11,15,16). Newer diagnostic imaging modalities have played an important role in the establishment of conservative therapy as the appropriate choice in patients with PEA (14,17,18). Computerized tomography (CT) widespread use in the last years and its use as gold standard imaging test in diagnostic dilemmas of patients with acute abdominal pain has resulted in increased recognition and diagnosis of PEA (11,17). Radiologists and surgeons should be aware of the typical imaging findings of PEA in order to accurately diagnose this entity and avoid further non-indicated pharmaceutical or surgical management (1,19-21).
Anatomy
Epiploic appendages consist of pedunculated fat tissue attached to the colonic surface, most commonly located on the taeniae (taenia libera and taenia omentalis) of the cecum and sigmoid colon (22,23). They are approximately 50 to 100 and form two lines along the colonic surface with the exception of the transverse colon, where only one row exists due to attachment of the greater omentum to taenia omentalis (7,11,15-17). These outpouchings are covered by serosa, supplied by one or two arteries and one draining vein and their length varies between 0.5–5 cm (11,23,24). Epiploic appendages first develop during the fifth month of intrauterine life and their size remains small during childhood (7,15,25). They get enlarged during adulthood and this size increase is augmented in obese patients, thus PEA is more frequently diagnosed in obese adults (7,15,25). The exact functional role of epiploic appendages is not well-understood and many different physiological roles have been proposed (17,24,26). It has been theorized that these fat projections act as cushions providing mechanical protection or as a storage of blood supply during peristalsis and colonic vessels compression (24,26). Other theories include the role as a fat storage utilized in periods of decreased caloric intake—starvation or immune protection and defensive mechanisms against inflammation, a role similar to that mediated by greater omentum (17,24,26).
Pathogenesis
Epiploic appendages, in the context of their anatomic structure of a bulbous protrusion connected to a narrow peduncle, undergo torsion with consequent vascular supply impairment, initially affecting the venous component (11,15,27). Ischemia of the epiploic appendages may also occur as a result of draining vein thrombosis (5,6). Both conditions lead to edema, ischemic necrosis, aseptic inflammation of the affected appendage and eventually absorption by the peritoneal cavity (5-7,15). Nevertheless, Virchow was the first to suggest that detached loose intraperitoneal bodies represent detached epiploic appendages (28). In the era of laparoscopic surgery and radiographic diagnosis, calcified detached epiploic appendages can be identified as peritoneal loose bodies, also known as ‘peritoneal mice’ (29,30).
PEA should always be differentiated from secondary epiploic appendagitis (SEA), an entity that results from a different pathophysiologic mechanism. SEA involves inflammation of a normal epiploic appendage located in proximity to an inflamed organ, such as colon (diverticulitis), appendix (appendicitis) or gallbladder (cholecystitis) (15,31,32). The most frequent source of inflammation in SEA is diverticulitis and pathognomonic signs of the adjacent organ disease are evident in diagnostic imaging modalities, such as CT (31,32).
Clinical and laboratory findings
The clinical presentation of PEA is vague and similar to those caused by other acute conditions such as acute appendicitis (right lower quadrant abdominal pain), acute diverticulitis (left lower quadrant abdominal pain) and acute cholecystitis (right upper quadrant abdominal pain) (Tables 1,2) (6,9,11,22,33,34). PEA most commonly manifests with acute onset, non-migrating lower abdominal pain, most commonly on the left, with localized tenderness on abdominal palpation and rebound tenderness in some occasions (2,11,24,35). Fever, nausea, vomiting, diarrhea and constipation are sometimes associated with PEA, but are usually absent (2,8,35). Mollà et al. demonstrated that approximately 7% of patients undergoing investigation to exclude the initial presumptive clinical diagnosis of acute diverticulitis of the sigmoid colon showed imaging findings of PEA (23). Choi et al., in their retrospective case series study of 31 patients with PEA, reported abdominal tenderness (100%) and right or left lower quadrant abdominal pain (41.9% and 41.9%, respectively) as the most common presenting symptoms in their cohort, whereas muscle rigidity and fever were absent in all patients (22). They also reported that patients with sigmoid diverticulitis were older than patients with PEA affecting sigmoid colon appendages (69.7 vs. 41.4 respectively, P<0.001) (22). Other conditions, presenting with symptoms similar to PEA, include pelvic inflammatory disease, ovarian torsion, ectopic pregnancy, mesenteric lymphadenitis, acute omental infarction, mesenteric panniculitis and ureter stone (19,22,33). Laboratory evaluation in patients with PEA is usually within normal limits and the findings, if present, are mostly non-specific (Table 1) (8). In most cases erythrocyte sedimentation rate, liver transaminases, pancreatic amylase and lipase and urinalysis are within normal limits (5,15,18). Infrequently, a slightly elevated white blood cell count (WBC) and C-reactive protein, as a result of ischemic fat necrosis induced inflammatory response, may be observed (6,24,35).

Full table

Full table
Imaging studies
The lack of specific pathognomonic clinical and laboratory findings as well as absence of awareness among physicians renders PEA diagnosis difficult without the use of imaging modalities (2,33). Prior to establishment of newer diagnostic techniques, PEA was a diagnosis considered after the exclusion of more common diseases and was identified during diagnostic laparotomy or laparoscopy (5,15,36).
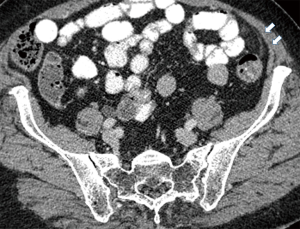
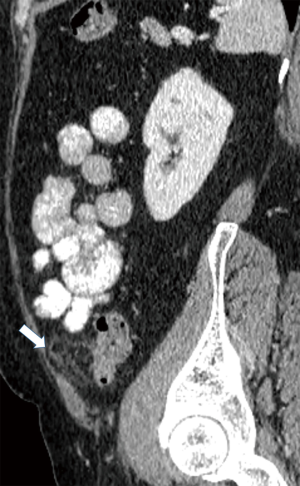
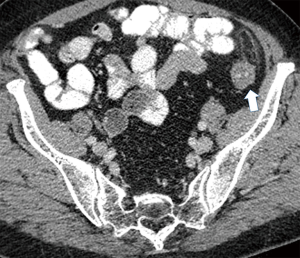
Currently, in the era of widespread use of CT scans in the differentiation of acute abdominal pain, PEA is easily identified in the presence of pathognomonic imaging findings and its reported incidence is increasing (Table 3) (22). The hallmark of PEA in CT consists of an ovoid mass measuring between 1.5 and 3.5 cm in maximal diameter with fat attenuation, surrounded by a hyperdense ring corresponding to the inflammatory reaction of the overlying serosa (visceral peritoneum) (Figure 1) (6,11). Frequently, the serosal surface spreads the inflammation through attachment to the parietal peritoneum, which may have a thickened appearance (Figure 2) (11,37). The occasional presence of a centrally located hyperdense area corresponds to the draining vein thrombosis (‘central dot sign’) (Figure 3) (6,8,11,37). Additionally, the anterior relationship of the inflamed epiploic appendage to the colonic wall is a useful anatomic imaging clue in order to confirm PEA diagnosis (Figure 4) (38). Nugent et al. reported that the ovoid mass with hyperattenuating ring (100%), the central hyperdense dot sign (79%), peritoneal thickening (76%), bowel wall thickening (Figure 5) (47%), presence of diverticula (28%) and free fluid were the most common imaging findings of PEA (10).

Full table
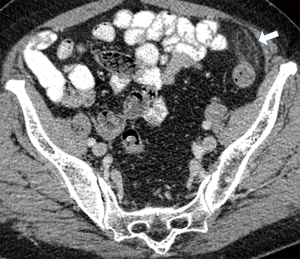
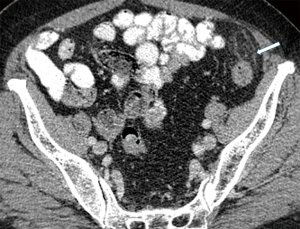
Ultrasound (U/S) at the site of maximal tenderness reveals an oval hyperechoic, non-compressible mass adjacent to the colonic surface (8,11,18,23,39). Doppler images reveal absence of central blood flow and the mass may be surrounded by a hypoechoic peripheral rim corresponding to thickened—inflamed serosal surface (7,8,12,18,24). The adjacent fat tissue may present with increased echogenicity and color Doppler signal in the context of inflammation induced increase in blood flow (7,15). In addition, U/S easily identifies the fixed attachment of inflamed appendages to the anterior abdominal wall during breathing movements (7,23,40). U/S is a rapid noninvasive imaging diagnostic test, usually helpful in the diagnosis of PEA in non-obese patients or those who have contraindications to CT radiation exposure, such as pregnant women (18).
Magnetic resonance imaging (MRI) is not a routinely used imaging test in the diagnosis of PEA, but can be used in pediatric patients and pregnants (41-43). Advantages of MRI include absence of ionizing radiation and higher soft tissue resolution in contrast to CT. Imaging findings include the presence of an oval mass with fat tissue signal intensity in T1 and T2 weighted MRI images and ring enhancement in contrast agent (gadolinium) enhanced T1 weighted imaging (11,15,41).
Treatment
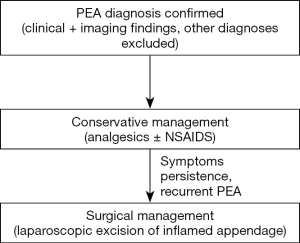
Prior to the widespread use of newer imaging diagnostic modalities, PEA was considered a surgical disease and was usually diagnosed and treated during operations performed to exclude more severe cause of acute abdomen (1,7,15) (Figure 6). Currently, PEA is generally considered a self-limiting disease and conservative management with or without NSAIDS is the first choice of treatment, resulting in disappearance of symptoms within several days, but is associated with a high rate of recurrence (2,8,12,24,33,40). Antibiotic usage has been proposed as an adjunct to anti-inflammatory medications, but their therapeutic benefit is not established (11,15,16,28). Nevertheless, if conservative approach fails to alleviate symptomatology, laparoscopic excision of the affected appendage may be required (8,15,24). Most patients treated conservatively show resolution of symptoms within 1–2 weeks, but the CT findings may persist and subside in a slower fashion (7,15,44). Furthermore, CT pathognomonic changes may persist for up to 6 months and physicians should be aware of the long-term imaging residual findings, in order to avoid misdiagnosis of patients presenting with acute abdominal pain due to other causes (38). PEA complications include abscess development and gastrointestinal obstruction; thus patients are suggested to immediately seek medical evaluation upon worsening of post-discharge clinical status (35).
Conclusions
PEA is an uncommon and frequently misdiagnosed cause of acute abdominal pain in patients presenting in the emergency department. Widespread CT usage has resulted in increased recognition and diagnosis of PEA. Upon establishment of diagnosis, PEA patients are treated with analgesics. Persistence of symptoms or recurrent episodes are treated with laparoscopic appendage excision. Surgeons and emergency department physicians should be aware of this frequently underdiagnosed entity.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- de Brito P, Gomez MA, Besson M, et al. Frequency and epidemiology of primary epiploic appendagitis on CT in adults with abdominal pain. J Radiol 2008;89:235-43. [Crossref] [PubMed]
- Ortega-Cruz HD, Martínez-Souss J, Acosta-Pumarejo E, et al. Epiploic Appendagitis, an Uncommon Cause of Abdominal Pain: A Case Series and Review of the Literature. P R Health Sci J 2015;34:219-21. [PubMed]
- Boscarelli A, Frediani S, Ceccanti S, et al. Magnetic resonance imaging of epiploic appendagitis in children. J Pediatr Surg 2016;51:2123-5. [Crossref] [PubMed]
- González-García A, Escribano-Pérez M, Diz Fariña S. Clinical Image In Gastroenterology: Epiploic appendagitis in an 80-year-old woman, an uncommon cause of acute abdominal pain in the elderly. Rev Gastroenterol Mex 2015;80:276-7. [PubMed]
- Son HJ, Lee SJ, Lee JH, et al. Clinical diagnosis of primary epiploic appendagitis: differentiation from acute diverticulitis. J Clin Gastroenterol 2002;34:435-8. [Crossref] [PubMed]
- Gourgiotis S, Oikonomou C, Veloudis G, et al. The Diagnostic Dilemma of Primary Epiploic Appendagitis and How to Establish a Diagnosis. Oman Med J 2016;31:235-7. [Crossref] [PubMed]
- Hollerweger A, Macheiner P, Rettenbacher T, et al. Primary epiploic appendagitis: sonographic findings with CT correlation. J Clin Ultrasound JCU 2002;30:481-95. [Crossref] [PubMed]
- Nadida D, Amal A, Ines M, et al. Acute epiploic appendagitis: Radiologic and clinical features of 12 patients. Int J Surg Case Rep 2016;28:219-22. [Crossref] [PubMed]
- Plummer R, Sekigami Y, Chen L, et al. Epiploic Appendagitis Mimicking Recurrent Diverticulitis. Case Rep Surg 2018;2018:1924067. [Crossref] [PubMed]
- Nugent JP, Ouellette HA, O’Leary DP, et al. Epiploic appendagitis: 7-year experience and relationship with visceral obesity. Abdom Radiol (NY) 2018;43:1552-7. [Crossref] [PubMed]
- Singh AK, Gervais DA, Hahn PF, et al. Acute Epiploic Appendagitis and Its Mimics. RadioGraphics 2005;25:1521-34. [Crossref] [PubMed]
- Chu EA, Kaminer E. Epiploic appendagitis: A rare cause of acute abdomen. Radiol Case Rep 2018;13:599-601. [Crossref] [PubMed]
- Quaas AM, Mueller PR, Kickham JM. Epiploic appendagitis mimicking pelvic inflammatory disease (PID). Eur J Obstet Gynecol Reprod Biol 2008;140:134-5. [Crossref] [PubMed]
- Sandrasegaran K, Maglinte DD, Rajesh A, et al. Primary epiploic appendagitis: CT diagnosis. Emerg Radiol 2004;11:9-14. [Crossref] [PubMed]
- Schnedl WJ, Krause R, Tafeit E, et al. Insights into epiploic appendagitis. Nat Rev Gastroenterol Hepatol 2011;8:45-9. [Crossref] [PubMed]
- Blinder E, Ledbetter S, Rybicki F. Primary epiploic appendagitis. Emerg Radiol 2002;9:231-3. [Crossref] [PubMed]
- Giambelluca D, Cannella R, Caruana G, et al. CT imaging findings of epiploic appendagitis: an unusual cause of abdominal pain. Insights Imaging 2019;10:26. [PubMed]
- Hasbahceci M, Erol C, Seker M. Epiploic appendagitis: is there need for surgery to confirm diagnosis in spite of clinical and radiological findings? World J Surg 2012;36:441-6. [Crossref] [PubMed]
- van Breda Vriesman AC, de Mol van Otterloo AJ, Puylaert JB. Epiploic appendagitis and omental infarction. Eur J Surg 2001;167:723-7. [Crossref] [PubMed]
- Ng KS, Tan AGS, Chen KKW, et al. CT features of primary epiploic appendagitis. Eur J Radiol 2006;59:284-8. [Crossref] [PubMed]
- Patel VG, Rao A, Williams R, et al. Cecal epiploic appendagitis: a diagnostic and therapeutic dilemma. Am Surg 2007;73:828-30. [PubMed]
- Choi YU, Choi PW, Park YH, et al. Clinical Characteristics of Primary Epiploic Appendagitis. J Korean Soc Coloproctol 2011;27:114. [Crossref] [PubMed]
- Mollà E, Ripollés T, Martínez MJ, et al. Primary epiploic appendagitis: US and CT findings. Eur Radiol 1998;8:435-8. [Crossref] [PubMed]
- Sand M, Gelos M, Bechara FG, et al. Epiploic appendagitis – clinical characteristics of an uncommon surgical diagnosis. BMC Surg 2007;7:11. [Crossref] [PubMed]
- Matsunaga H, Fujii Y, Taniguchi N. Ultrasonographic findings in epiploic appendagitis. J Med Ultrason (2001) 2010;37:31-2. [Crossref] [PubMed]
- Ghahremani GG, White EM, Hoff FL, et al. Appendices epiploicae of the colon: radiologic and pathologic features. Radiographics 1992;12:59-77. [Crossref] [PubMed]
- Rioux M, Langis P. Primary epiploic appendagitis: clinical, US, and CT findings in 14 cases. Radiology 1994;191:523-6. [Crossref] [PubMed]
- Talukdar R, Saikia N, Mazumder S, et al. Epiploic Appendagitis: Report of Two Cases. Surg Today 2007;37:150-3. [Crossref] [PubMed]
- Obaid M, Gehani S. Deciding to Remove or Leave a Peritoneal Loose Body: A Case Report and Review of Literature. Am J Case Rep 2018;19:854-7. [Crossref] [PubMed]
- Borg SA, Whitehouse G, Griffiths G. A mobile calcified amputated appendix epiploica. AJR Am J Roentgenol 1976;127:349-50. [Crossref] [PubMed]
- Osada H, Ohno H, Watanabe W, et al. Multidetector computed tomography diagnosis of primary and secondary epiploic appendagitis. Radiat Med 2008;26:582-6. [Crossref] [PubMed]
- Jalaguier A, Zins M, Rodallec M, et al. Accuracy of multidetector computed tomography in differentiating primary epiploic appendagitis from left acute colonic diverticulitis associated with secondary epiploic appendagitis. Emerg Radiol 2010;17:51-6. [Crossref] [PubMed]
- Bunni J, Corrigan A, Jacob K, et al. Epiploic appendagitis: A case report highlighting correlation between clinical features, computed tomography images and laparoscopic findings. Int J Surg 2010;8:401-3. [Crossref] [PubMed]
- Rao PM, Rhea JT, Wittenberg J, et al. Misdiagnosis of primary epiploic appendagitis. Am J Surg 1998;176:81-5. [Crossref] [PubMed]
- Akinosoglou K, Kraniotis P, Thomopoulos K, et al. Epiploic appendagitis: a non-surgical cause of acute abdomen. Ann Gastroenterol 2015;28:296-8. [PubMed]
- Carmichael DH, Organ CH. Epiploic disorders. Conditions of the epiploic appendages. Arch Surg 1985;120:1167-72. [Crossref] [PubMed]
- Almeida RR, Singh AK, Mansouri M, et al. Impact of Radiology Report Wording on Care of Patients With Acute Epiploic Appendagitis. AJR Am J Roentgenol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Singh AK, Gervais DA, Hahn PF, et al. CT appearance of acute appendagitis. AJR Am J Roentgenol 2004;183:1303-7. [Crossref] [PubMed]
- Vázquez-Frias JA, Castañeda P, Valencia S, et al. Laparoscopic diagnosis and treatment of an acute epiploic appendagitis with torsion and necrosis causing an acute abdomen. JSLS 2000;4:247-50. [PubMed]
- Lee YC, Wang HP, Huang SP, et al. Gray-scale and color Doppler sonographic diagnosis of epiploic appendagitis. J Clin Ultrasound 2001;29:197-9. [Crossref] [PubMed]
- Sirvanci M, Balci NC, Karaman K, et al. Primary epiploic appendagitis: MRI findings. Magn Reson Imaging 2002;20:137-9. [Crossref] [PubMed]
- Lo Re G, Carcione P, Vernuccio F, et al. Primary epiploic appendagitis in a pediatric patient: prominent role of Magnetic Resonance Imaging in the diagnosis. Minerva Pediatr 2015;67:529-30. [PubMed]
- Yang L, Jia M, Han P. Primary epiploic appendagitis as an unusual cause of acute abdominal pain in a middle-aged male: A case report. Medicine (Baltimore) 2019;98:e16846. [Crossref] [PubMed]
- Kani KK, Moshiri M, Bhargava P, et al. Extrahepatic, Nonneoplastic, Fat-Containing Lesions of the Abdominopelvic Cavity: Spectrum of Lesions, Significance, and Typical Appearance on Multidetector Computed Tomography. Curr Probl Diagn Radiol 2012;41:56-72. [Crossref] [PubMed]


