
Surveillance and management for serous cystic neoplasms of the pancreas based on total hazards—a multi-center retrospective study from China
Introduction
Pancreatic serous cystic neoplasm (SCN) was first reported concurrently by Hodgkinson et al. (1) and Compagno and Oertel (2) in 1978. SCN usually appears as a great number of small agminated cysts in a honeycomb arrangement and is abundant in clear, glycogen-rich cells (3); and consists of 10–16% of pancreatic cystic tumor (PCN) cells (4). With rapid advances in cross-sectional imaging technology, SCN is frequently detected, and is commonly thought to be a benign tumor. However, the majority of SCN easily confused in clinical practice with PCN, like mucinous cystic neoplasm (MCN), intraductal papillary mucinous neoplasm (IPMN), and solid pseudopapillary tumor (SPT) that are potentially malignant (5,6). Consensus is still lacking as to whether SCN is necessary for surgical treatment or surveillance strategies.
Considering the potential risk of malignancy and mechanical complications, some centers insist that surgical treatment is necessary for SCN (7,8). Additionally, a few of these SCN patients may have turn out to have pre-malignant or malignant lesions after surgical resection. On the contrary, other medical centers only recommend resection according to guidelines on patients with SCN-relative symptoms or uncertain cyst diagnosis (9). Primary cyst size, macrocystic or oligocystic variant type, growth rate, age and history of other malignancies have been consistently proposed to predict the malignancy of SCN in recent clinical management (6,10). The ideal time point for surgical treatment hasn’t yet been established which may lose the optimal opportunity for SCN resection.
In addition, SCN patients undergoing surgical resection may suffer from complications, such as severe pancreatic fistula (PF), delayed gastric emptying (DGE) and so on, the probability of surgical mortality is up to 1.0–2.7% in high-volume centers (11,12). Thus, it is critical to accurately differentiate between SCNs and other non-SCNs to provide proper treatment decisions and maintain a balance between the risk of malignancy and surgical treatment. Previous multi-center research reported that surgical treatment of SCN should be proposed in a minority of patients and only for uncertain diagnoses remaining after complete workup including computed tomography (CT) scan, magnetic resonance imaging (MRI) scan and endoscopic ultrasonography (EUS), significant and related symptoms or exceptionally when concerned that a malignancy exists (6).
However, in some low-income cities or low-volume centers, CT and MRI scans are used instead of EUS due to institutional inexperience with EUS technology so that precise preoperative diagnosis is highly difficult. There is a remarkable trend for other types of PCN to be misdiagnosed with SCN, which may significantly increase the risk of malignancy. Therefore, in our study, two cohorts of SCN patients from multi-centers were analyzed to compare the total risk of malignancy due to radiological misdiagnosis with risk of surgical mortality. This analysis indicated that preoperative radiological diagnosed SCN with limited examinations still has the potential to be administered with surgical treatment.
Methods
Patient population and data source
SCN patients who underwent curative resection in 16 Chinese institutions between January 2006 and December 2016 were retrospectively enrolled in this study. Enhanced abdominal CT or MRI scans were routinely performed before operation. According to their radiological data, number of lesions and tumor characteristics of maximum diameter, mural nodule, oligocystic or polycystic, enhancing cyst wall, separation, calcification and solid mass were all included in our analysis. The intraoperative situation, postoperative pathology and complications were recorded as well. However, owing to a retrospective essence and total usage of EUS biopsy was less than 20% among all institutes at that time, patients with EUS biopsy before surgery were excluded in this study. Additionally, patients were divided into two cohorts. One cohort included all patients confirmed to have SCN with postoperative pathological analysis, and the other cohort contained all patients diagnosed as SCN with preoperative MRI or CT scan. All SCN patients preoperatively diagnosed with radiology were done in accordance with its radiological characteristics, and all indications for surgery were considered by the attending doctors according to the current domestic guideline at the time and hospital protocol. The ethics committee of Zhongshan Hospital, Fudan University approved this study (B2014-019).
CT and MRI
CT and MRI scans were individually read by radiologists with over 10 years of experience and by pancreatic surgeons with over 5 years of experience to enhance the reliability of imaging diagnosis. The final report was determined after both the radiologist and pancreatic surgeon had separately analyzed the images and then come to an agreement.
Definition of postoperative complications
PF was defined in accordance with criteria published in 2005 by the International Study Group on Pancreatic Fistulas (ISGPF). DGE can be divided into grade A, B, and C according to the definition of the International Study Group on Pancreatic Surgery and sometimes needed to be confirmed by endoscopy or an upper gastrointestinal gastrografin series.
Statistical analysis
All statistical analyses were performed with SPSS 21.0 statistical package (SPSS Inc., Chicago, IL, USA) for Windows. Correlations and differences between categorical or continuous variables were analyzed with Pearson Chi-squared test, Fisher’s Exact test or Student’s t-test. A P value <0.05 was considered as a statistically significant difference.
Results
Clinical characteristics of SCN patients with postoperative pathological diagnosis
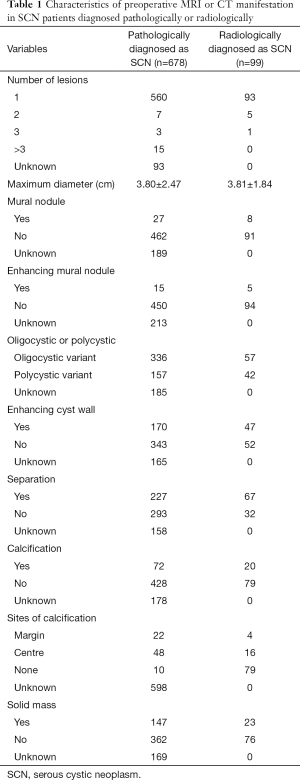
From our multi-center data, 678 patients were finally pathologically confirmed as having SCN after resection. As revealed in their preoperative MRI or CT scan (Table 1), 649 patients (95.7%) had only one lesion and the average of maximum diameter was 3.80±2.47 cm. Among the whole patients, 27 patients (5.5%) manifested mural nodule, while 15 patients showed enhancing mural nodule. Then 336 patients (68.2%) had oligocystic variant, while 31.8% of patients had polycystic variant. The wall enhancement of cyst existed in 170 patients (33.1%), cystic separation in 227 patients (43.7%), calcification in 72 patients (14.4%) and solid mass in 147 patients (28.9%).
Full table
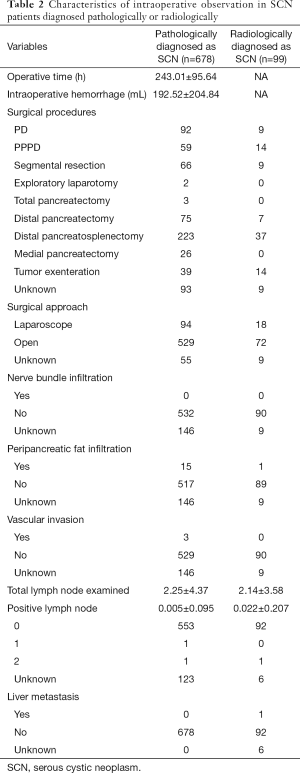
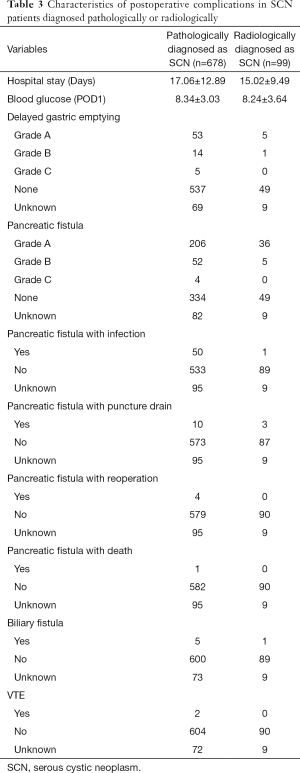
The average of operative time was 243.01±95.64 hours and the average of intraoperative hemorrhage volume was 192.52±204.84 mL. In addition, the optimal surgical procedure was chosen to excise the tumor lesion and 529 patients (84.9%) underwent open surgery. After pathologic analysis, we found that no patients had nerve bundle infiltration or liver metastasis, while 15 patients (2.8%) had peripancreatic fat infiltration, three patients (0.6%) had vascular invasion and two patients (0.4%) had lymph node metastasis (Table 2). The average hospital stay was 17.06±12.89 days and the percentage of patients with DGE was 11.8% and five of those patients suffered from DGE of grade C after surgery. PF occurred in 262 patients (44.0%) and four patients had grade C PF, as shown in Table 3. Of 678 patients, only four patients had serous cystic carcinoma (SCC), so the actual malignancy rate of SCN was approximately 0.6%.
Full table
Full table
Clinical characteristics of radiologically diagnosed SCN
Considering to its low malignancy rate, we further analyzed 99 patients diagnosed with SCN after preoperative MRI or CT scan. We found 93.9% of patients had only one lesion and its average maximum diameter was 3.81±1.84 cm. Other radiological characteristics are listed in Table 1. Among the whole group, 72 patients (80.0%) accepted open surgery. Only one patient had a positive lymph node exam after pathological examination (Table 2); their average hospital stay was 15.02±9.49 days. The percentage of patients with DGE was up to 6.7%, but none had DGE of grade C after surgery. A total of 41 patients had postoperative PF, as shown in Table 3. However, among these patients, three patients were diagnosed with IPMN, nine with MCN and four with SPT after postoperative pathological examination. The radiological characteristics of these sixteen non-SCN patients showed that enhancing cyst wall was found in five patients, oligocystic variant in eleven patients, separation of cyst in ten patients, calcification in three patients and solid mass in three patients.
Correlation between preoperative radiological characteristics and SCN diagnosis
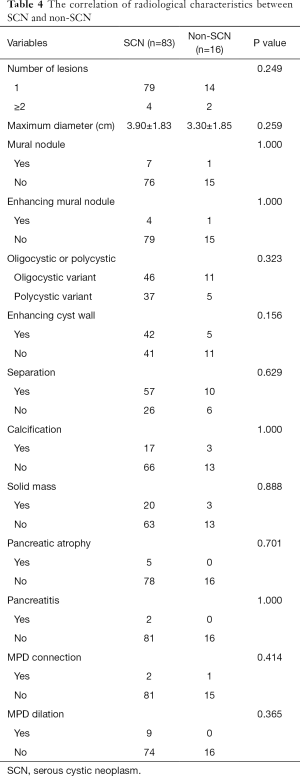
A total of 99 patients diagnosed with SCN before surgery had diverse radiological characteristics. For example, 16.16% of patients were diagnosed with non-SCN post-operatively via pathological test. To improve diagnostic accuracy preoperatively, we further analyzed the preoperative radiological characteristics between SCN and non-SCN. However, no significant difference in characteristics were found between SCN and non-SCN, including lesion numbers, maximum diameter, mural nodule, oligocystic or polycystic variant, cyst wall, separation, calcification, solid mass, pancreatic atrophy, pancreatitis, MPD connection and MPD dilation (Table 4). Therefore, the exact differential diagnosis of SCN by traditional radiological methods before surgery remains difficult.
Full table
Development of risk prediction formula
Through a recent survey in China, it showed the malignancy rate of IPMN was nearly 32.1%, MCN was about 10.4% and SPT was approximately 12.3% (13). Thus, we constructed a novel formula, total theoretical risk of malignancy of SCN diagnosis = [32.1%X + 10.4%Y + 12.3%Z + 0.6% (n − X − Y − Z)]/n (X, number of pathological IPMN diagnosis; Y, number of pathological MCN diagnosis; Z, number of pathological SPT diagnosis; n, total patients with preoperative SCN diagnosis). Within the imaging diagnosis of SCN before surgery, the tumors were confirmed by the finial pathological examination as IPMN, MCN, SPT and SCN after surgical resection. However, the different risks of malignancy in these cystic tumors were stated in our previous study (13). So based on risk weight of final pathological diagnosis, this formula was established to calculate the theoretical malignant risk. Through this formula, we calculated a theoretical malignant risk index of 2.9%, which was higher than the surgical mortality of nearly 0.2–2% in these high-volume centers. Taken together, our results show that surgical resection should be undertaken depending on limited radiological diagnosis and detailed surgical plan in SCN.
Discussion
Pancreatic cancer deserves more attention as a precancerous lesion that is remarkably malignant with a limited 5-year overall survival (14,15). Pancreatic cysts are differentiated based on their malignant potential, and SCN virtually never progresses to an invasive lesion (16). Currently, however, the management of SCN remains controversial. In our study, the actual malignancy rate of SCN with postoperative pathological diagnosis was just 0.6%, which was lower than the mortality rate of pancreatic surgery. However, SCN diagnosis was inaccurate before surgery as 16 out of 99 SCN patients were finally pathologically diagnosed as non-SCNs. According to their separate malignant potential, the total theoretical malignancy rate of radiological SCN diagnosis was up to 2.9% which was higher than pancreatic surgical mortality. Thus, considering the malignancy rate and surgical mortality, the management of SCN deserves a more careful diagnostic check.
Difficulties in obtaining a precise radiological diagnosis preoperatively, symptoms resulting from SCN involvement, and risk of malignant transformation are considered to be the main arguments for surgical resection (7,8). In contrast, the severe morbidity and mortality of surgery and tumor indolence are powerful arguments supporting a more conservative approach. There is still no consensus on the ideal treatment approach for diagnosed SCN.
Some medical centers selected patients for surgery according to tumor size and growth rates. In a small single center study, tumors less than 4 cm had a slower growth rate than tumors greater than or equal to 4 cm (17). Growth rate was confirmed to be significantly higher for tumors no less than 4 cm in a recent study (6). According to the Japan Pancreas Society, SCN larger than 4 cm was an indication for surgical resection, and pathological findings including papillary proliferation, nuclear atypia, venous invasion, peripancreatic fat tissue infiltration, and lymph vessel invasion were indicative of malignant SCN (3). In addition, some studies reported that symptomatic SCN was also a suitable indicator for surgery, a tumor which was responsible for the symptoms (18).
Some surgeons have advocated for an active, early surgical strategy for the improvement of pancreatic surgery and risk of complications of an asymptomatic SCN (7). This strategy hinted at the essentiality of evaluating the risk-benefit balance between surgery and follow-up. In some high-volume centers, mortality of surgical strategy was much lower than reported. Furthermore, long-term and short-term morbidity of pancreatic surgery remained high with PF, DGE, diabetes mellitus and/or exocrine pancreatic insufficiency (11,19-21). Therefore, all treatment options should be carefully considered.
Among our data, only 13.7% of SCN patients were diagnosed correctly before surgery at that time, and 55.8% were given only a suggested PCN diagnosis. One reason for the low accuracy was preoperative diagnosis in China employed only MRI or CT scan for routine examination far more than EUS. However, once casually increased preoperative SCN diagnosis at such level, the misdiagnosis rate of other PCNs may severely increase. So only we absolutely make sure the SCN diagnosis, then the misdiagnosis rate may decrease. Clinicians should keep in mind that increased misdiagnosis rate is usually accompanied by increased rough diagnosis of SCN in common volume hospital.
Accurate diagnosis before surgery seemed to be much more significant. If SCN patients can be accurately diagnosed with radiological tests, many of them can avoid pancreatic surgery. On CT scan, SCNs are generally polycystic, and although a sunburst calcification is pathognomonic, only 11% to 30% of tumors are apparent (22,23). MRI scanning can reveal accurate visualizations of the lesions’ structure, in particular the presence of septa; but this method lacks sensitivity to calcifications. Magnetic resonance cholangiopancreatography (MRCP) is more popular that gives a better evaluation of spatial relationship between the pancreatic or biliary duct and the lesions to discriminate the differential diagnosis. The absence of communication with the Wirsung duct allows a certain diagnosis of SCN (24). EUS was preliminarily reported to have 82.93% accuracy when used to determine the differential diagnosis between SCN and MCN (5). In addition, previous researches have reported on clinical features and molecular marker panels that showed promise for the accurate classification of PCN and identification of cysts that required surgical treatment in the future (16,25).
Conclusions
We described the specific mortality and natural history of SCNs using a large Chinese cohort from multiple expert centers. Our findings suggested that SCN was almost a completely benign lesion with a malignancy rate was just 0.6%. However, considering its potential misdiagnosis, the total malignancy rate went up to 2.9%, which was higher than the risk of surgical mortality. Thus, a surgical strategy should be considered in a minority of patients based on the patient volume at the institution, surgical skills available and patient conditions. When SCN can’t be accurately distinguished from cystic tumors of pancreas, the risk of malignance of cystic tumors may be higher than surgical risk. However, if it can be diagnosed as SCN correctly like using EUS, SCN should not be performed with surgery as well. Only when we pay more attention to such a benign tumor can risk balance between malignancy and surgical mortality be achieved.
Acknowledgments
Funding: The authors thank Yadong Xu (Zhongshan Hospital, Fudan University) for data collections. This work was supported by the following grants: Shanghai Municipal Population and Family Planning Commission (2013SY053), Zhongshan Clinical Trial (2016ZSLC14) and Clinical Science and Technology Innovation Project, Shenkang Hospital Development Center of Shanghai (SHDC12017X04).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee of Zhongshan Hospital, Fudan University approved this study (B2014-019).
References
- Hodgkinson DJ. ReMine WH, Weiland LH. Pancreatic cystadenoma. A clinicopathologic study of 45 cases. Arch Surg 1978;113:512-9. [Crossref] [PubMed]
- Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol 1978;69:289-98. [Crossref] [PubMed]
- Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012;41:380-7. [Crossref] [PubMed]
- Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery 2012;152:S4-12. [Crossref] [PubMed]
- Zhang W, Linghu E, Chai N, et al. New criteria to differentiate between mucinous cystic neoplasm and serous cystic neoplasm in pancreas by endoscopic ultrasound: A preliminarily confirmed outcome of 41 patients. Endosc Ultrasound 2017;6:116-22. [Crossref] [PubMed]
- Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016;65:305-12. [Crossref] [PubMed]
- Hwang HK, Kim H, Kang CM, et al. Serous cyst adenoma of the pancreas: appraisal of active surgical strategy before it causes problems. Surg Endosc 2012;26:1560-5. [Crossref] [PubMed]
- Strobel O. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion 2003;68:24-33. [Crossref] [PubMed]
- Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339-49. [Crossref] [PubMed]
- Malleo G, Bassi C, Rossini R, et al. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut 2012;61:746-51. [Crossref] [PubMed]
- Pulvirenti A, Marchegiani G, Pea A, et al. Clinical Implications of the 2016 International Study Group on Pancreatic Surgery Definition and Grading of Postoperative Pancreatic Fistula on 775 Consecutive Pancreatic Resections. Ann Surg 2018;268:1069-75. [Crossref] [PubMed]
- Birnbaum DJ, Gaujoux S, Berbis J, et al. Surgery for pancreatic neoplasms: How accurate are our surgical indications? Surgery 2017;162:112-9. [Crossref] [PubMed]
- Pancreatic Surgery of Chinese Academic Society of Young Surgeons. The current status of diagnosis and treatment of pancreatic cystic neoplasm in China: a report of 2 251 cases. Zhonghua Wai Ke Za Zhi 2018;56:24-9. [PubMed]
- Pu N, Lv Y, Zhao G, et al. Survival prediction in pancreatic cancer patients with no distant metastasis: a large-scale population-based estimate. Future Oncol 2018;14:165-75. [Crossref] [PubMed]
- Pu N, Gao S, Xu Y, et al. Alkaline Phosphatase-To-Albumin Ratio as a Prognostic Indicator in Pancreatic Ductal Adenocarcinoma after Curative Resection. J Cancer 2017;8:3362-70. [Crossref] [PubMed]
- Carr RA, Yip-Schneider MT, Dolejs S, et al. Pancreatic Cyst Fluid Vascular Endothelial Growth Factor A and Carcinoembryonic Antigen: A Highly Accurate Test for the Diagnosis of Serous Cystic Neoplasm. J Am Coll Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg 2005;242:413-9; discussion 419-21. [PubMed]
- Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013;45:703-11. [Crossref] [PubMed]
- Kondo N, Murakami Y, Uemura K, et al. Prognostic impact of postoperative complication after pancreatoduodenectomy for pancreatic adenocarcinoma stratified by the resectability status. J Surg Oncol 2018;118:1105-14. [Crossref] [PubMed]
- Krautz C, Nimptsch U, Weber GF, et al. Effect of Hospital Volume on In-hospital Morbidity and Mortality Following Pancreatic Surgery in Germany. Ann Surg 2018;267:411-7. [Crossref] [PubMed]
- Buanes TA. Role of surgery in pancreatic cancer. World J Gastroenterol 2017;23:3765-70. [Crossref] [PubMed]
- Antonini F, Fuccio L, Fabbri C, et al. Management of serous cystic neoplasms of the pancreas. Expert Rev Gastroenterol Hepatol 2015;9:115-25. [Crossref] [PubMed]
- Warshaw AL, Compton CC, Lewandrowski K, et al. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg 1990;212:432-43; discussion 444-5. [Crossref] [PubMed]
- Gourgiotis S, Germanos S, Ridolfini MP. Presentation and management of pancreatic cystic neoplasms. J Clin Gastroenterol 2007;41:599-608. [Crossref] [PubMed]
- Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501-10. [Crossref] [PubMed]


