
Phenotypic characterization of CADASIL patients with the Arg332Cys mutation in the NOTCH3
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a dominant, single-gene, hereditary cerebrovascular disease, characterized by premature migraine (20–30%), recurrent subcortical ischemic stroke (60–80%), subcortical dementia, and affective disturbance (30%) (1,2). Noticeably, a few patients present with cerebral microbleeding, epileptic seizure, Parkinson’s syndrome, alopecia, or osphyalgia. On brain magnetic resonance imaging (MRI), patients with CADASIL typically show bilateral symmetric diffused leukoaraiosis accompanied by multiple lacunar infarcts in the subcortical and periventricular white matter, bilateral basal ganglia, and external capsule. T2/FLAIR hyperintensity in the temporal pole (O’Sullivan’s sign) (3), external capsule, and corpus callosum is the characteristic manifestation of CADASIL. Currently, genetic testing is considered to be the “gold standard” for diagnosing CADASIL. Mutations in exons 2-24 of the NOTCH3 gene on chromosome 19 have been identified as the cause behind the pathogenesis of CADASIL (4). To date, more than 200 different mutations have been reported in the NOTCH3 gene. Interestingly, these mutations tend to cluster in exons 4, 3, and 11 (5).
In 2001, Oliveri and coworkers (6) first detected the Arg332Cys mutation in exon 6 of two patients with CADASIL in an Italian family, who mainly presented with recurrent ischemic episodes. However, to our knowledge, the Arg332Cys mutation in exon 6 has been rarely reported in patients with CADASIL. In this study, we report a patient with CADASIL carrying the Arg332Cys mutation. We also performed a comprehensive literature review and sought to summarize the clinical manifestations and neuroimaging characteristics of patients with this disease.
Methods
Case report
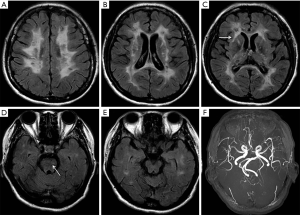
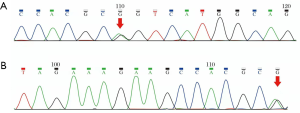
A 48-year-old woman presented with rapidly progressive dementia and urinary incontinence within 6 months. She had a history of recurrent episodes of ischemic stroke for 5 years, albeit without regular traditional vascular risk factors, including hypertension, diabetes, smoking, alcoholic, hyperhomocysteinemia, and heart disease. Neurological examination revealed diffuse brisk deep tendon reflexes and positive Babinski sign on both sides of the body. The performance on Mini-mental State Examination was severely impaired, with a score of 3/30, and the results of laboratory tests were unremarkable. The results of 24-h Holter monitoring, carotid ultrasound, and echocardiography were normal. Brain MRI revealed high-intensity signals in the cerebral white matter, bilateral thalamus, internal and external capsules, basal ganglia, corpus callosum, and brainstem on FLAIR (Figure 1A,B,C,D). No obvious abnormalities were detected in the temporal pole (Figure 1E) or on magnetic resonance angiography (Figure 1F). The symptoms and results of brain MRI indicated the possible diagnosis of CADASIL. The patient underwent a genetic test for the NOTCH3 gene, and a nucleotide variation of c.994G>A was identified (Figure 2A) in exon 6 that caused a missense mutation (Arg332Cys). The same mutation (Figure 2B) was detected in her healthy 21-year-old daughter.
Literature review
Articles on multicenter retrospective studies and case reports in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) were searched using the keywords “CADASIL”, “Arg332Cys”, “R332C”, and “exon 6”. All the articles and important references thus retrieved were studied in depth, and the relevant information was extracted and summarized. We obtained 9 articles that reported the presence of the Arg332Cys mutation in 11 European patients and 10 Asian patients (6-14). Of the 11 European patients, 9 were excluded due to the lack of clinical and imaging data, and 2 Italian patients and 9 Asian patients were considered for our study. We selected the largest published case series on the clinical manifestations of 536 patients from Europe, America, and Asia (15) with complete clinical and imaging data, for comparing and analyzing the clinical and neuroimaging features of CADASIL patients carrying the Arg332Cys mutation in exon 6.
Results
Overall, the Arg332Cys mutation is present in a low proportion of the reported CADASIL cases. In 48 British families with CADASIL, the pathogenic mutations in exon 6 was present in only 6% of the cases (7). Razvi and coworkers (8) reported that 69.1% of the mutations in NOTCH3 in 39 Dutch families with CADASIL were harbored in exons 3 or 4, while only 5.1% of the mutations were in exon 6. Another study (9) on 120 German patients with CADASIL, who showed positive results in genetic testing, reported that exons 4, 3, and 11 were the most frequent mutation sites, harboring 67.5% of the total number of mutations, while only 2.5% of the mutations were present in exon 6. In 2005, Tang (10) reported the first Asian mutation of Arg332Cys in a Taiwanese family. A subsequent study (11) on 21 Taiwanese patients with CADASIL demonstrated that the pathogenic mutations were primarily mapped to exon 11 (47.6%) and exon 4 (19%), while the Arg332Cys mutation in exon 6 was observed in only 1 patient. Recently, a multicenter retrospective study involving 214 Chinese patients with CADASIL demonstrated that the same mutation was present in only 2.38% of the patients (14). To date, the Arg332Cys missense mutation in exon 6 of the NOTCH3 gene has not been included in Exon Aggregation Consortium and Human Genetic Variation Database.
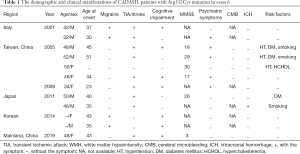
The demographic and clinical features of the 12 CADASIL patients, selected from our report and literature search, carrying the Arg332Cys mutation in exon 6 of NOTCH3, are enlisted in Table 1. The patients comprised 7 men and 5 women, of which 10 were from Asia. The average age at initial symptom onset was 37.82±9.36 years (ranging from 23 to 51 years), which was earlier than 57.0±9.36 years reported in the study by Pescini (15). Of the patients, 16.7% had only one of the four cardinal symptoms of CADASIL, including migraine, transient ischemic attack (TIA)/stroke, cognitive impairment, or psychiatric symptoms, while the majority of the patients presented with two or more symptoms. The most frequent manifestations were TIA or stroke (83.3%), followed by cognitive impairment (58.3%) and psychiatric symptoms (50%, including depression, mania, and behavioral changes), similar to the results of the study by Pescini (15), where patients suffered TIA or stroke accounted for 72% and cognitive impairment accounted for 43%. Migraine accounted for a third of the total 12 patients and was observed in 20% of the 10 Asian patients considered herein (Tables 1,2). The prevalence of migraine in European patients was 60.9%, compared to only 8.5% in Asian patients, as reported in literature (15) (Table 2). In this study, detailed neuroimaging data of the 2 Italian patients (6) were unavailable. The remaining 10 patients were from Asia, and the findings of neuroimaging are enlisted in Table 3. All the patients exhibited diffuse white matter hyperintensities on FLAIR. Characteristic high anterior temporal signal was observed in 6 patients (60%, exceeding the 34.5% reported in the study by Pescini (15) on Asian patients). The external capsule and brainstem high signals were observed for 9 (90%) and 5 (71.43%) patients, respectively. Cerebral microbleeding was not observed in the 6 Asian patients with available gradient echo imaging data. Only 1 patient developed intracranial hemorrhage. The morbidity of intracranial hemorrhage in patients with Arg332Cys mutation (8.3%) was lower than that (25%) reported in a previous study (16).
Full table

Full table

Full table
Discussion
In this study, we report a 48-year-old woman with a rare Arg332Cys mutation in exon 6 of the NOTCH3 gene, who presented with rapidly developing dementia and recurrent ischemic stroke. Based on a comprehensive literature review, we further summarized the clinical and imaging features of CADASIL patients carrying the Arg332Cys mutation.
The clinical presentations of patients with the Arg332Cys mutation are similar to the classical symptoms of CADASIL, with the exception of a few variations. First, the age of onset of CADASIL in the patients with the Arg332Cys mutation is 20 years earlier than that of the patients without the Arg332Cys mutation, on an average. Secondly, several studies have demonstrated that the incidence of migraine in Asian patients is much lower (5%) than that in European patients (43%) (17). However, 20% of Asian patients with the Arg332Cys mutation have been reported to suffer from migraines. Thirdly, intracranial hemorrhage and cerebral microbleeding are relatively uncommon in European patients with CADASIL, but their incidences have been reported to be higher in Asian patients, with the incidences in European and Asian patients being 25% and 55%, respectively. In our study, cerebral microbleeding was not observed in the 6 patients with available neuroimaging data. Only 1 patient with hypertension suffered intracranial hemorrhage (Table 1). Fourth, studies (15) on general CADASIL patients without the Arg332Cys mutation have reported that the prevalence of high signals of the bilateral temporal pole is lower in Asian patients (34.5%) than in European patients (86%). The incidence of high signals of the bilateral temporal pole in the 10 Asian patients considered in our study was higher, being 60% (Table 2). Additionally, 71.43% of the patients with the Arg332Cys mutation exhibited brainstem hyperintensity on FLAIR (Table 2), which is also a common feature in the Asian CADASIL patients without the Arg332Cys mutation (18). Nearly all the patients with the Arg332Cys mutation possessed severe white matter lesions as revealed by MRI, with multiple lacunar cerebral infarcts involving the bilateral basal ganglia and external capsule.
Conclusions
CADASIL patients carrying the Arg332Cys mutation in exon 6 have been reported from Europe and Asia, with the majority of patients having early disease onset. Diffuse high signals involving the external capsule, brainstem, and bilateral temporal pole are the main neuroimaging characteristics.
Acknowledgments
The authors express their sincere gratitude to the participant for her help and willingness to participate in this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted after receiving written informed consent from the patient.
References
- Chabriat H, Vahedi K, Iba-Zizen MT, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet 1995;346:934-9. [Crossref] [PubMed]
- Sourander P, Wålinder J. Hereditary multi-infarct dementia. Morphological and clinical studies of a new disease. Acta Neuropathol 1977;39:247-54. [Crossref] [PubMed]
- O’sullivan M, Jarosz JM, Martin RJ, et al. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology 2001;56:628-34. [Crossref] [PubMed]
- Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996;383:707-10. [Crossref] [PubMed]
- Hassan A, Markus HS. Genetics and ischemic stroke. Brain 2000;123:1784-812. [Crossref] [PubMed]
- Oliveri RL, Muglia M, De Stefano N, et al. A Novel Mutation in the Notch3 Gene in an Italian family with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy: Genetic and Magnetic Resonance Spectroscopic Findings. Arch Neurol 2001;58:1418-22. [Crossref] [PubMed]
- Markus HS, Martin RJ, Simpson MA, et al. Diagnostic strategies in CADASIL. Neurology 2002;59:1134-8. [Crossref] [PubMed]
- Razvi SS, Davidson R, Bone I, et al. Diagnostic strategies in CADASIL. Neurology 2003;60:2019-20. [Crossref] [PubMed]
- Peters N, Opherk C, Bergmann T, et al. Spectrum of Mutations in Biopsy-Proven CADASIL: Implications for Diagnostic Strategies. Arch Neurol 2005;62:1091-4. [Crossref] [PubMed]
- Tang SC, Lee MJ, Jeng JS, et al. Arg332Cys mutation of NOTCH3 gene in the first known Taiwanese family with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neurol Sci 2005;228:125-8. [Crossref] [PubMed]
- Lee YC, Liu CS, Chang MH, et al. Population-specific spectrum of NOTCH3 mutations, MRI features and founder effect of CADASIL in Chinese. J Neurol 2009;256:249-55. [Crossref] [PubMed]
- Sano Y, Shimizu F, Kawai M, et al. p.Arg332Cys mutation of NOTCH3 gene in two unrelated Japanese families with CADASIL. Intern Med 2011;50:2833-8. [Crossref] [PubMed]
- Kim YE, Yoon CW, Seo SW, et al. Spectrum of NOTCH3 mutations in Korean patients with clinically suspicious cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging 2014;35:726.e1-6. [Crossref] [PubMed]
- Chen S, Ni W, Yin XZ. Clinical features and mutation spectrum in Chinese patients with CADASIL: A multicenter retrospective study. CNS Neurosci Ther 2017;23:707-16. [Crossref] [PubMed]
- Pescini F, Nannucci S, Bertaccini B, et al. The Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) scale: a screening tool to select patients for NOTCH3 gene analysis. Stroke 2012;43:2871-6. [Crossref] [PubMed]
- Zhu S, Nahas SJ. CADASIL: Imaging Characteristics and Clinical Correlation. Curr Pain Headache Rep 2016;20:57. [Crossref] [PubMed]
- Liem MK, Oberstein SA, van der Grond J, et al. CADASIL and migraine: A narrative review. Cephalalgia 2010;30:1284-9. [Crossref] [PubMed]
- Wang Z, Yuan Y, Zhang W, et al. NOTCH3 mutations and clinical features in 33 mainland Chinese families with CADASIL. J Neurol Neurosurg Psychiatry 2011;82:534-9. [Crossref] [PubMed]


