
Initial anticoagulation experience with standard-dose rivaroxaban after Watchman left atrial appendage occlusion
Introduction
Percutaneous left atrial appendage occlusion (LAAO) has been increasingly used as an alternative to lifelong oral anticoagulation (OAC) for thromboprophylaxis in atrial fibrillation (AF) patients who suffer increased bleeding risk or have a contraindication to OAC (1). The satisfying results of PROTECT-AF and PREVAIL trials have fueled the USA and Europe approval of the Watchman device for LAAO to reduce the risk of AF-associated thromboembolism (2,3). Post-LAAO antithrombotic regimen should be applied to prevent device-related thrombosis (DRT), which is pivotal during the initial 45 days after LAAO until ingrowing endothelium covers the surface on device. Current antithrombotic protocol accepted for the Watchman device is warfarin and aspirin for 45 days, dual antiplatelet therapy (DAPT) for 6 months and aspirin for life (4). However, the need for frequent monitoring, narrow therapeutic range, dietary restrictions and multiple drug interactions associated with warfarin could result in unsatisfied compliance and low time in therapeutic range (TTR) at the beginning of 45-day’s usage, which contributes to the prevalence of non-vitamin K oral anticoagulants (NOACs) (5). Rivaroxaban, directly targeting to Xa factors, represents an alternative to warfarin in AF due to preferable trade-off between embolism and bleeding. In ROCKET-AF trial, rivaroxaban assigned to 20 mg was associated with similar rates of stroke and systemic embolism (SE) in comparison to warfarin, in reverse with lower rates of intracranial and fatal hemorrhage (6). Given that, rivaroxaban might be a feasible and safe regimen to prevent DRT, thromboembolic and bleeding events after LAAO with the Watchman device. In this study, we reported our single-center experience with the first 10 Chinese patients allocated to rivaroxaban 20 mg for post-LAAO antithrombotic therapy.
Methods
Study population
All consecutive patients who underwent successful Watchman device implantation between October 1, 2016 and September 30, 2017 at our institution were enrolled in a retrospective database. Only patients who received rivaroxaban in the periprocedural period were included in the current study. Indications for LAAO were non-valvular AF with CHA2DS2-VASC score ≥2 and HAS-BLED score ≥3, and were deemed to be poor candidates for long-term OAC. Exclusion criteria were mechanical heart valve, left ventricular ejection fraction <30%, intracardiac thrombus, and end-stage renal disease [estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2]. The physician (Ben He) who performed the Watchman device implantation have attended a rigorous training and achieved the certification to ensure the appropriate level of expertise. All patients signed informed consent for the procedure and data collection.
Procedure, follow-up, and outcomes
The detail of a Watchman device implantation procedure can be found elsewhere (7). Briefly, the procedure was performed with the patient under general anesthesia and intravenous heparin was given to achieve a target activated clotting time (ACT) of >250 seconds. The device was implanted with transesophageal echocardiography (TEE), left atrial appendage (LAA) angiography, and fluoroscopic guidance via right femoral vein and transseptal access. The TEE and LAA angiography were used to rule out LAA thrombosis and determine suitable device size. After the procedure, the sheath was removed and vascular access hemostasis was achieved with manual compression. Patients were planned to receive rivaroxaban 20 mg daily for at least 45 days after LAAO, followed by DAPT for 6 months and aspirin for long-term. TEE follow-up was scheduled at 6 weeks, at 6 months, and at 12 months post-implantation to detect DRT or peri-device leak, following a consensus proposed by two senior ultrasound physicians (Zhi-Qing Qiao and Heng Ge). DRT was defined as the detection of a thrombus formation adherent to the luminal (left atrial) side of the device by TEE scan. Meanwhile, thromboembolic and bleeding events were also evaluated at the time of follow-up. Thromboembolic complications included stroke, transient ischemic attack (TIA), and SE, while bleeding events were classified as major bleeding (a decrease in hemoglobin level of 2 g/dL or greater within a 24-hour period, or leading to a transfusion of 2 or more units of packed red cells, or requiring an additional endoscopy intervention or surgical operation) and minor bleeding according to international society on thrombosis and hemostasis criteria (8).
Statistics
Statistical analyses were performed using the SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA). Numbers are presented as median with interquartile range (IQR) for continuous variables and as percentage for categorical variables. Inferential statistic including survival analysis is not performed due to limited sample size and low incidence of DRT.
Results
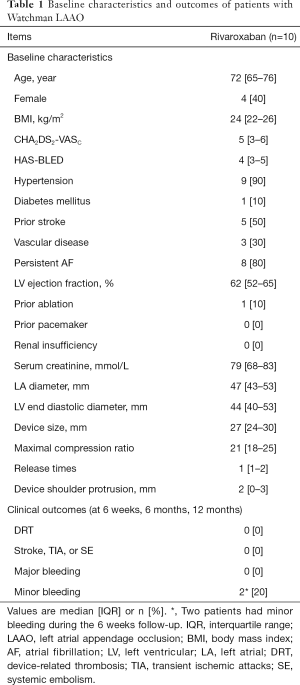
A total of 57 AF patients underwent successful Watchman device implantation and received either rivaroxaban or warfarin post-LAAO at our institution. Of which, 10 patients receiving rivaroxaban in the periprocedural period were included. Baseline characteristics of the study patients are outlined in Table 1. These cases had high risks of stroke and bleeding, with the median CHA2DS2-VASC score of 5 and HAS-BLED score of 4. The most common risk factors for stroke were hypertension (9 patients) and a history of ischemic stroke (5 patients). Permanent AF was present in 8 patients. All the patients completed a planned 45-day regimen of rivaroxaban, and no DRT, peri-device leakage, thromboembolic complications, and major bleeding were observed at time of follow-up (6 weeks, 6 months, and 12 months) (Table 1, Figure 1). One patient experienced a minor epistaxis and another had a gingival bleeding and ecchymosis on left arm during the 6 weeks follow-up, and without rivaroxaban discontinuation. Therefore, insights from the above finding, rivaroxaban seemed to be a suitable antithrombotic agent after the Watchman device implantation.
Full table

Discussion
Current guidelines from ESC and AHA/ACC/HRS recommend LAAO in AF patients who are at high risk of bleeding or with a contraindication to OAC (1,9). As the direct exposure of the thrombogenic device to the left atrial blood flow, DRT risk and its adverse clinical sequelae are the main concerns early after device implantation, as endothelialization on the device is still uncompleted. In PROTECT-AF trial, the overall incidence of DRT was initially found to be 4.2% (20/478) (2). And in a re-analysis that mainly investigated patients with suspected DRT, 5.6% (27/485) of patients experienced DRT, with warfarin (1.4%) at 45-day as compared to subsequent DAPT (3.9%) at 6 months and aspirin alone (2.5%) at 12 months, suggesting that the use of anticoagulants early after Watchman implant is antithrombotic effective and pivotal (10). Although warfarin still remains a very common agent used in the clinical setting, it is being fast replaced by NOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) with the reason of narrow therapeutic window, the need for frequent monitoring, and multiple food and drug interactions. All these issues conspire to increase the complexity of warfarin usage and extend the time of reaching an adequate therapeutic range from initiation. By contrast, NOACs is rapid onset and could quickly achieve and maintain a therapeutic level for 6 weeks, which is important for early anticoagulation for DRT prevention. Up to now, several studies have assessed the feasibility and safety of NOAC use after LAAO. In a multicenter retrospective analysis that involved 214 patients allocated to NOAC (46% apixaban, 46% rivaroxaban, 7% dabigatran, and 1% edoxaban), thrombus formation on device was detected in 2 (0.9%) patients in the NOAC group and 1 (0.5%) patient in the warfarin group. The rates of DRT therefore were comparable between NOACs and warfarin treatment (P=1.0) (11). Recently, Bergmann et al. (12) reported the 3-month TEE data from EWOLUTION study; 10.8% (109/1005) of patients received standard- or reduced-dose NOAC and had the numerically lowest rate of major bleeding in comparison to warfarin (1.9% vs. 2.0%) or DAPT (1.9% vs. 2.4%), without attenuating the efficacy to prevent DRT (1.3%) when compared to DAPT (1.3% vs. 3.1%). Nevertheless, evidences suggested stroke prevent property and major bleeding risk varied across NOACs (13), and standard-dose NOACs were safer and effective in Asians than in non-Asians (14). Our study is the first to evaluate the feasibility and safety of standard-dose rivaroxaban use after Watchman device implant in China. In this study, none of the patients experienced DRT, peri-device leak, thromboembolic complications and major bleeding events during 12 months follow-up, which is line with the above landmark studies (11,12).
Appropriate antithrombotic therapy could significantly reduce the development of DRT. However, DRT is also influenced by many factors, such as device type (Watchman or ACP), patient characteristics (older age, prior ischemic stroke, left ventricular dysfunction) and drug reaction (clopidogrel non-responders) (15,16). Nitinol cage and nitinol plug devices may have different thrombogenicity profiles and endothelialization processes, and different antithrombotic management may be required for these 2 devices (16). Ketterer et al. identified two-thirds of patients presenting with DRT after LAAO as clopidogrel non-responders, suggesting that drug reaction of patients is a critical factor in DRT prevention and DAPT may not be effective for clopidogrel non-responders post-LAAO (17). Therefore, a tailored post-procedural antithrombotic strategy should be determined with careful consideration of above influence factors.
There are inherent limitations in this study. Firstly, this is a retrospective, observational, single-center study. Secondly, inferential statistic including survival analysis may not be meaningful and were not calculated due to limited sample size and low event rate of DRT. Finally, although TEE is a gold standard for detecting the left atrial thrombosis, small thrombus on the device may not be observed.
The finding is important for clinical practice because NOACs are being increasingly applied owing to their safety and efficacy profiles. This study described our initial experience of standard-dose rivaroxaban as periprocedural anticoagulation regimen with Watchman device implantation. The results suggested that a short course of rivaroxaban at the dosage of 20 mg appeared to come with a good balance between thrombotic complications and major bleeding events, which may be a viable alternative following Watchman LAAO. In the era of NOAC, further randomized trials and large sample of real-world studies would be necessary to confirm the superiority of rivaroxaban after LAAO.
Acknowledgments
Funding: This study was supported by Natural Science Foundation of China (81770238), Research Funds of Shanghai Health and Family Planning commission (20184Y0022), Cultivation fund of clinical research of Renji Hospital (PY2018-III-06), Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001, CXYJY2019QN004) and Program for Key but Weak Disciplines of Shanghai Municipal Commission of Health and Family Planning (2016ZB0304).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this study. This study was approved by Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (No. 2018-030).
References
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988-98. [Crossref] [PubMed]
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. [Crossref] [PubMed]
- Shen L, Fang SS, Ge H, et al. Optimal nonvitamin K antagonist oral anticoagulant therapy in a warfarin-sensitive patient after left atrial appendage closure: A case report. Medicine (Baltimore) 2018;97:e0683. [Crossref] [PubMed]
- Wei AH, Gu ZC, Zhang C, et al. Increased risk of myocardial infarction with dabigatran etexilate: fact or fiction? A critical meta-analysis of over 580,000 patients from integrating randomized controlled trials and real-world studies. Int J Cardiol 2018;267:1-7. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]
- Saw J, Lempereur M. Percutaneous left atrial appendage closure: procedural techniques and outcomes. JACC Cardiovasc Interv 2014;7:1205-20. [Crossref] [PubMed]
- Schulman S, Angerås U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202-4. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Main ML, Fan D, Reddy VY, et al. Assessment of device-related thrombus and associated clinical outcomes with the WATCHMAN left atrial appendage closure device for embolic protection in patients with atrial fibrillation (from the PROTECT-AF trial). Am J Cardiol 2016;117:1127-34. [Crossref] [PubMed]
- Enomoto Y, Gadiyaram VK, Gianni C, et al. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm 2017;14:19-24. [Crossref] [PubMed]
- Bergmann MW, Betts TR, Sievert H, et al. Safety and efficacy of early anticoagulation drug regimens after WATCHMAN left atrial appendage closure: three-month data from the EWOLUTION prospective, multicentre, monitored international WATCHMAN LAA closure registry. EuroIntervention 2017;13:877-84. [Crossref] [PubMed]
- Zhou LY, Yang SF, Zhang Z, et al. A renal function based trade-off analysis of non-vitamin k antagonist oral anticoagulants in nonvalvular atrial fibrillation. Front Physiol 2018;9:1644. [Crossref] [PubMed]
- Wang KL, Lip GY, Lin SJ, et al. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke 2015;46:2555-61. [Crossref] [PubMed]
- Kubo S, Mizutani Y, Meemook K, et al. Incidence, characteristics, and clinical course of device-related thrombus after watchman left atrial appendage occlusion device implantation in atrial fibrillation patients. JACC Clin Electrophysiol 2017;3:1380-6. [Crossref] [PubMed]
- Fauchier L, Cinaud A, Brigadeau F, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol 2018;71:1528-36. [Crossref] [PubMed]
- Ketterer U, D’Ancona G, Siegel I, et al. Percutaneous left atrial appendage occlusion: Device thrombosis in clopidogrel non-responders. Int J Cardiol 2016;204:196-7. [Crossref] [PubMed]


