Oral prednisolone and triamcinolone injection for gastric stricture after endoscopic submucosal dissection
Introduction
The expansion of the indications for endoscopic submucosal dissection (ESD) to include early gastric cancers has enabled extensive resection (1,2). However, post-ESD stenosis after large resections applied to the gastric cardia or pylorus is often difficult to manage, requiring multiple endoscopic balloon dilatation (EBD) (3). Iizuka et al. reported the frequency of post-ESD stenosis as 1.9% of all gastric ESD; Tsunada et al. reported 0.9%; and Nonaka et al. reported 1.0% (4-6). However, they reported that 17% of patients who underwent ESD for gastric cardia cancer had post-ESD stenosis, and 7% of patients who underwent ESD for pyloric cancer had post-ESD stenosis (6). Coda et al. reported the risk factors for post-ESD stenosis for the gastric cardia or pylorus; a resection of more than three-fourths of the circumference of the gastric cardia or pylorus is a significant factor related to stenosis (3). Again, all patients who had post-ESD stenosis were symptomatic (3). Dysphagia was the main symptom related to post-ESD stenosis in cases of gastric cardia cancer, whereas nausea or vomiting was most common for pyloric lesions (3). The median symptomatic period was 22 (range, 16-33) days and 27 (range, 15-46) days for gastric cardia and pyloric cancer, respectively (3). On the other hand, post-ESD stenosis occurs rarely following ESD for lesions of the body of the stomach (7). Thus, the risk of post-ESD stenosis varies depending on resection size and location.
Indeed, EBD has been a treatment option for stenosis after large ESD, but EBD is sometimes complicated by perforation (8-10). In addition, repeat EBD substantially compromises patients’ quality of life. Therefore, a new treatment strategy for post-procedural stenosis is necessary for large gastric ESD. In this regard, endoscopic triamcinolone injection and oral prednisolone have shown promising results for the prevention of luminal stricture following extensive esophageal ESD (11,12). The aim of the present study was to evaluate the benefit of oral prednisolone and triamcinolone injection for stenosis after extensive gastric ESD.
Methods
Patients
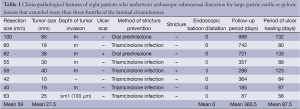
A total of eight patients (all men; mean age, 68.5 years; range 42-85 years, mean follow-up period, 360.5 days; range 87-989 days) who underwent large ESD was enrolled in this study. The clinicopathological features of each patient are summarized in Table 1. Written informed consent was obtained from each patient before ESD. The tumor was demarcated using indigocarmine or crystal violet staining to determine resection size.
Full table
Management of stricture after ESD
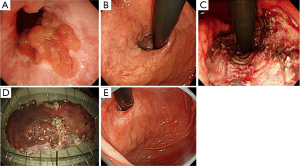
Large ESD was defined as ESD done for lesions that extended more than three-fourths of the luminal circumference of the gastric cardia or pylorus in accordance with previous studies (3,4,7). Four patients underwent ESD for gastric cardia cancer, and four patients were treated for pyloric lesions. Steroid (oral prednisolone or endoscopic triamcinolone injection) was administered to prevent stenosis after ESD in these cases. In two patients with gastric cardia cancer, oral prednisolone was started at 30 mg daily on the second day after complete circular ESD. Prednisolone was then tapered gradually (daily 30, 25, 20, 15, 10, and 5 mg for 14 days each) and discontinued at 12 weeks. In six patients (two gastric cardia lesions, four pyloric lesions), topical injection of 80 mg triamcinolone via endoscopy was performed once immediately after ESD using the short-type needle (N-type, Top Co, Tokyo, Japan; Figure 1) for safe injection onto the post-ESD ulcer. Triamcinolone injection has sometimes caused esophageal perforation with the conventional endoscopic needle, and, hence, the length of the needle should be shorter than the conventional needle (1.8 vs. 4.0 mm for the novel and conventional needles, respectively), to achieve safe application of gastric triamcinolone injections.

Follow-up endoscopy was performed one week, two weeks, and one month after ESD, and continued once a month until ulcer healing (epithelialization of the post-ESD ulcer). The patients were then followed at six months and one year after ESD, and annually thereafter.
EBD was used in cases with stricture-related symptoms or signs including nausea, vomiting, or food residuals observed on endoscopy. EBD was also used if a 10-mm-diameter endoscope could not be passed through the lumen. The incidence of stenosis, the frequency and period required for EBD, the duration required for ulcer healing after ESD, and the incidences of post-procedural bleeding and perforation were assessed. Post-procedural bleeding was defined as bleeding that required transfusion or bleeding that caused the hemoglobin level to fall 2 g/dL (2). Post-procedural perforation was diagnosed based on the presence of free air on abdominal computed tomography (13).
Results
As shown in Table 1, there were six IIa and two IIc macroscopic types according to the Paris endoscopic classification (14). The mean resection size was 59 (range, 40-100) mm, and the mean tumor size was 27.5 (range, 10-85) mm. As for tumor invasion depth, there were six intramucosal carcinomas, one slightly invasive carcinoma into the submucosa, and one adenoma. The mean period of ulcer healing was 87.5 (range, 56-133) days. One of the eight patients had post-ESD stenosis that required EBD, the median frequency and period required for EBD was 0 times and 0 days (range, 0-3 times, 0-74 days), respectively. The median ulcer healing period after ESD was 87.5 (range, 56-133) days, and no patient experienced post-procedural bleeding or perforation. There were no adverse events in association with steroid therapy.
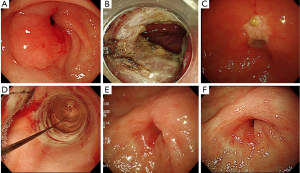
The patient who had complete circular gastric cardia lumen resection for early gastric cancer and was given oral prednisolone for 12 weeks did not develop post-ESD stenosis (case No. 1, Figure 2). However, the patient who had complete circular ESD for early gastric cancer in the pylorus and was given topical triamcinolone injection developed post-ESD stenosis (case No. 5). EBD and additional triamcinolone injection were added, but the next week, a 10-mm-diameter scope could not pass again, and only EBD was added, because triamcinolone generally remains 3-4 weeks in the local area. Twelve weeks after ESD, a 10-mm-diameter endoscope could not pass again, and EBD and a triamcinolone injection were added in combination with oral prednisolone starting at 30 mg/day. Prednisolone was then tapered gradually (daily 30, 25, 20, 15, 10, and 5 mg for 14 days each) and discontinued at 12 weeks. After the combination therapy, the patient remained free from stricture for about one year (Figure 3).
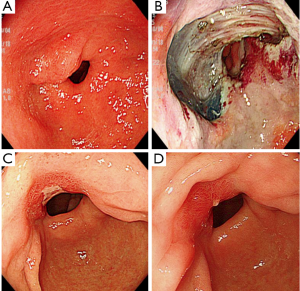
On the other hand, the remaining six patients who underwent semicircular large ESD did not develop post-ESD stenosis with either triamcinolone injection (representative case No. 7, Figure 4) or oral prednisolone.
Discussion
ESD enables curative en bloc removal of gastrointestinal neoplasms irrespective of size in early non-invasive stage of the diseases (1,2). However, post-ESD stricture has become a major concern, particularly with superficial esophageal epithelial neoplasms (15). Esophageal stricture causes dysphagia and aspiration pneumonia and decreases patients’ quality of life (11,15,16). Similarly, post-ESD stenosis causes symptoms such as dysphagia in cases of large ESD for gastric cardia cancer, and nausea or vomiting for pyloric cancer (6). Indeed, EBD has been a treatment option for stenosis after large ESD, but EBD is sometimes complicated by perforation (8-10). In addition, repeat EBD substantially compromises patients’ quality of life, even in large gastric ESD. In fact, Nonaka et al. reported the median frequency and period required for EBD without prevention was 5 (range, 1-14) times and 42 (range, 1-120) days, respectively, for large gastric cardia lesions and 9 (range, 7-40) times and 50 (range, 28-198) days, respectively, for pyloric lesions (6).
Thus, a new treatment strategy for post-procedural stenosis is necessary for large gastric ESD. In this regard, endoscopic triamcinolone injection and oral prednisolone have shown promising results for the prevention of luminal stricture following extensive esophageal ESD (11). Isomoto et al. reported the usefulness of oral prednisolone administration for the management of post-procedural stricture even in cases of complete circular ESD for extensive esophageal epithelial neoplasms (12). There were some case reports on the successful management of post-ESD stricture with steroid injection onto a post-large gastric ESD ulcer for pyloric lesions (17,18). Few reports for gastric cardia lesions were seen. Coda et al. reported the risk factors for post-ESD stricture for gastric cardia or pyloric lesions, as described previously (3). According to their studies, the length of tumor resection was important; resection over 50 mm in size was a significant risk factor (cardia, P<0.05 and pylorus, P<0.01) (3). In the present study, six patients had resections over 50 mm. Only one patient who underwent a 58-mm-long ESD developed post-ESD stenosis, but the other patients did not post-ESD stenosis with prophylactic steroid therapy.
It took no less than almost three months until ulcer healing following large gastric ESD in our study. Steroid use might exacerbate peptic ulcers and has not been established as safe for post-ESD ulcers of the stomach. There were no adverse events with steroid therapy in the present small series. Further observation and larger studies are warranted to draw conclusions about the relationship between steroid use and ulcer healing and safety.
One patient had post-ESD stenosis following complete circular resection for a large pyloric early gastric cancer, which was not prevented by triamcinolone injection. The intractable stricture was ultimately resolved with oral prednisolone treatment. Another patient who underwent complete circular ESD for gastric cardia cancer did not develop stenosis with oral prednisolone. Although the location of ESD was different (between the cardia and pylorus), the present study suggests that oral prednisolone could be more useful than triamcinolone injection for complete circular resection, as shown in prior studies in esophageal ESD (11).
There have been new technologies for the management of esophageal stricture, such as autologous oral mucosal sheets, temporary stent insertion, and so on (19,20). However, few modalities have been used for gastric stricture so far, and the present therapeutic experience with oral prednisolone administration or triamcinolone injection for large gastric ESD suggests that they can be useful options to prevent gastric stricture or to reduce the number and adverse events of EBD sessions.
Conclusions
Oral prednisolone and triamcinolone injection were effective for the management of post-ESD stricture following large gastric ESD for gastric cardia and pyloric lesions without serious complications, indicating the safety and usefulness of the steroid treatment. Further evaluation in a large series is needed before steroid therapy is recognized as an option for the management of post-ESD stricture.
Acknowledgements
Authors’ contributions: Hiroyuki Shoji, acquisition of data, analysis and interpretation of data, conception and design, drafting the manuscript; Naoyuki Yamaguchi, acquisition of data, analysis and interpretation of data; Hajime Isomoto, drafting and revising the manuscript; Hitomi Minami, acquisition of data, analysis and interpretation of data; Kayoko Matsushima, acquisition of data, analysis and interpretation of data; Yuko Akazawa, drafting and revising the manuscript; Ken Ohnita, analysis and interpretation of data; Fuminao Takeshima, drafting and revising the manuscript; Saburo Shikuwa, analysis and interpretation of data; Kazuhiko Nakao, drafting and revising the manuscript, and general supervision of the research group. All authors read and approved the final manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007;10:1-11. [PubMed]
- Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54-8.
- Coda S, Oda I, Gotoda T, et al. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009;41:421-6. [PubMed]
- Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 2010;22:282-8. [PubMed]
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008;67:979-83. [PubMed]
- Nonaka S, Oda I, Abe S, et al. The management of gastrointestinal stenosis after wide-range ESD for early gastric cancer. Endoscopia Digestiva 2013;25:721-8.
- Hagiwara S, Onozato Y, Iizuka H, et al. Gastric stricture after endoscopic submucosal dissection for early gastric cancers. Progress of Digestive Endoscopy 2010;77:35-9.
- Yoda Y, Yano T, Kaneko K, et al. Endoscopic balloon dilatation for benign fibrotic strictures after curative nonsurgical treatment for esophageal cancer. Surg Endosc 2012;26:2877-83. [PubMed]
- Pereira-Lima JC, Ramires RP, Zamin I Jr, et al. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol 1999;94:1497-501. [PubMed]
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010;2:29-35. [PubMed]
- Yamaguchi N, Isomoto H, Shikuwa S, et al. Systemic and local steroid administration for the treatment of esophageal stricture after extensive endoscopic submucosal dissection: strategies and problems. Endoscopia Digestiva 2013;25:679-87.
- Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011;11:46. [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661-5. [PubMed]
- Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003;57:165-9. [PubMed]
- Mori H, Kobara H, Fujihara S, et al. Recanalization of severe gastric antral stricture after large endoscopic submucosal dissection: mucosal incision and local steroid injection. J Gastrointestin Liver Dis 2012;21:435-7. [PubMed]
- Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [PubMed]
- Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006;55:1704-10. [PubMed]
- Saito Y, Tanaka T, Andoh A, et al. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci 2008;53:330-3. [PubMed]


