Future perspective of gastric cancer endotherapy
Principle of endoscopic resection
Early gastric cancer (EGC) is defined when the cancer invasion is confined to the mucosa or submucosa (T1 cancer), regardless of the presence of lymph node metastasis (1). The five-year cancer-specific survival rates in patients who underwent gastrectomy with lymph node dissection for EGC limited to the mucosa or the superficial submucosa were 99% and 96%, respectively (2,3). Because the presence of lymph node metastasis is a strong predictor on patients’ prognosis (4). Considering reductions of quality of life after gastrectomy (5) and low risk of lymph node metastasis (up to 3%), surgery to remove intramucosal gastric cancer might be excessive for the majority of patients.
In comparison, surgery in the majority is appropriate when the cancer involves the deep submucosa where the incidence of lymph node metastasis increases to as high as 20% (6). A stratification method to identify patients who have negligible risk for developing lymph node metastasis would thus optimize the selection of patients who can be cured by endoscopic resection and thus avoid the risks of surgery. The ideal patients for endoscopic resection are those who have a lower mortality risk from metastasis as compared to that from surgery (7). Pathological staging would be the best predictor of the risk of lymph node metastasis (8,9). The major advantage of endoscopic resection is the ability to provide an accurate pathological staging without precluding future surgical therapy (10,11). After endoscopic resection, pathological assessment of depth of cancer invasion, degree of cancer differentiation and involvement of lymphatics or vessels allows the prediction of the risk of lymph node metastasis (12).
Endoscopic submucosal dissection (ESD) technique was developed to extend the ability of Endoscopic mucosal resection (EMR) to remove lesions en bloc larger than 2 cm (13,14), as EMR is limited to the resection of small tumors. It is also known that piecemeal resections in lesions >2 cm lead to a high-risk for local cancer recurrence and inadequate pathological staging (15,16). ESD allows for large “en bloc” resection regardless tumor size, location and/or submucosal fibrosis and allow a precise pathological staging (17-19). ESD is the most gratifying for patients with EGC because of its minimally invasive and curative potentials. It is increasingly used globally (20,21).
Indication for endoscopic resection
The traditional criteria for endoscopic resection of EGC were founded on the technical limitation of EMR to remove gastric lesions less than 2 cm in diameter en bloc. The empirical indications for EMR include (22): (I) papillary or tubular (differentiated) adenocarcinoma; (II) less than 2 cm in diameter; (III) without ulceration within tumor; (IV) no lymphatic-vascular involvement.
Clinical observations have noted however that the empirical indications for EMR were too strict and had led to unnecessary surgery. Therefore, expanded criteria for endoscopic resection have been proposed especially after large en bloc resection could be technically being accomplished using ESD (23). The large number of patients included in the study reported by Gotoda and colleagues was instrumental in defining the expanded criteria as its 95% confidence intervals (CI) were narrow (24). In a study involving 5,265 patients who had undergone gastrectomy with careful lymph node dissection and pathological analysis, the risks of lymph node metastasis can be clustered to a number of pathological findings of the involved mucosa and submucosa: macroscopic appearance, size, depth, differentiation of cancer, lymphatic and vascular involvement. This seminal work provides one of the pillars of endoscopic resection of EGC. In addition, recent data showed that no lymph node metastasis was found in 310 patients with poorly differentiated adenocarcinoma and/or signet-ring cell EGC, less than 2 cm in diameter, without ulceration and without lymphatics or vascular involvement (95% CI: 0-0.96%) (25). The current indication of endoscopic resection for patients with EGC today is called the Expanded Criteria for Endoscopic Resection in EGC (Table 1).
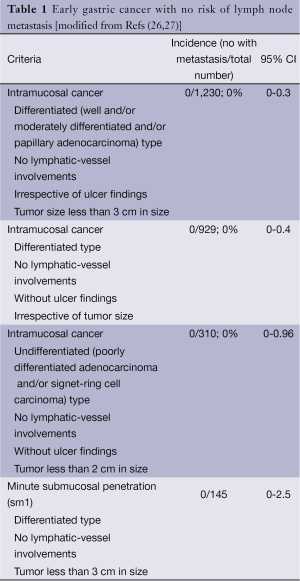
Full table
The Paris classification of superficial neoplasia of the gastrointestinal tract provides another pillar. It allows standardization of the endoscopic appearance of EGC, which is then useful to estimate tumor depth and likelihood of risk of lymph node metastasis (28). The en bloc resected specimen provides further information on size, depth, differentiation of cancer and lymphatic-vascular involvement through pathological assessment.
Outcomes of endoscopic resection
The long-term outcomes of patients who were treated by endoscopic resections have provided the ultimate proof of its safety and efficacy. The long-term outcomes after EMR for small differentiated mucosal EGC less than 2 cm in diameter have been reported to be comparable to those following gastrectomy (29-31). Patients who underwent ESD following the expanded criteria have similar long-term survival and outcomes as patients treated according to the traditional criteria (32). The 5-year survival rate was 92% in patients with traditional criteria group, 93% in the expanded criteria group. There was no significant difference in overall survival between both groups (multivariable-hazard ratio; 1.10, 95% CI 0.67-1.81).
The criteria are best used by comparing the risk of developing lymph node metastasis or distant metastasis against the risk of surgery and considering patients’ morbidities and preferences (26,27). It is very important to understand that the expanded criteria were developed to identify which patients have a low risk of lymph node metastasis. Patients meeting the criteria incur a risk of lymph node metastasis up to the upper limit of 95% CI.An (33,34).
Complications
Although delayed bleeding is thought as the most common complication occurring in up to 8% of patients undergoing gastric ESD (35). Acute bleeding may obscure the visual field, leading to a higher risk of complications. Therefore, endoscopic hemostasis should be immediately performed step by step. Small vessels can be coagulated using the ESD knife by forced coagulation mode, 50W (ESG-100, Olympus Medical Systems or ICC200, ERBE, Germany). Larger vessels should be coagulated by special designed hemostatic forceps (FD-410LR, Olympus Medical Systems) using soft coagulation, 80W (ESG-100, Olympus Medical Systems or ICC200, ERBE, Germany) (36).
After dissection has been completed, further hemostasis is performed on visible vessels to minimize delayed bleeding. The hemostatic forceps using soft coagulation mode is used to coagulate any visible small vessels (37). Excessive coagulation in the area of the exposed muscle layer of the ESD defect should be avoided because of the risk of delayed perforation. Delayed bleeding, manifested by hematemesis or melena at 0-30 days after the procedure, are treated by emergent endoscopy, performed after resuscitation (what does this mean?), using similar techniques (38).
Perforations are typically closed by endoclips (39). If pneumoperitoneum is significant, the patient may develop respiratory compromise or even shock. Thus, decompression of the pneumoperitoneum must be immediately performed. In order to prevent gastric perforations and facilitate ESD procedure recently polyethylene glycol or sodium hyaluronate as an injection agent have been reported. These agents remain longer in the submucosa and create a more clear dissection layer (40,41). The use of CO2 to insufflated the stomach during ESD is also extremely useful as CO2 is readily absorbed should perforation occurs. No evidence of peritoneal dissemination and/or lymph node metastasis caused by gastric perforation has been reported (42).
Training
The learning and application of these relatively complex endoscopic techniques for EGC has been shown across the world (43-46). Most Japanese experts set the level of expertise at 50-100 cases in order to become proficient in gastric ESD (47), requiring a trainee to perform at least 30 gastric ESD cases under the supervision of an expert to gain basic proficiency in this technique (48). Participation in hands-on courses with isolated or live animal stomach as well as live demonstrations is vital to accelerate the learning curve.
A standardized ESD training system is urgently needed to disseminate safe and effective ESD technique to practices with limited ESD experience. The panel concluded that preceptees should observe and attend ESD procedures as an assistant in at least 20 and 5 cases, respectively, in order to understand a wide variety of ESD procedures and strategies to develop trouble-shooting abilities (49). The trainee should start with antral lesion less than 20 mm in diameter without ulcer as it has the lowest risk of non-curative resection (50), and then progress to lesions in distal stomach and proximal stomach (51). Submucosal dissection has been shown to be more difficult than mucosal incision, mostly related to uncontrollable hemorrhage (52).
Western ESD experts to supervise ESD training are limited, and virtual simulators for ESD are not yet available. Thus, the use of animal models to facilitate the early training of ESD is important in order to minimize the higher complications at the beginning of the learning curve in humans. Proper use of ex vivo and in vivo animal models is performed in an animal facility under the direction of specialized veterinarian with dedicated equipment and standardized set up resulting in an effective learning strategy (53,54). A European group assessed the impact on 18 experienced endoscopists who participated in a 2-day training course that included seminars and hands-on training with living pigs, and was supervised by experts in ESD (55). The use of models allows endoscopists to ascend the learning curve in a relatively short time, and enhance the safety and efficacy of the patient experience. Finally, the technical expertise, training opportunities and backgrounds of endoscopist embarking on ESD in the West differ significantly than their Eastern counterparts (56,57).
Limitations of standard endoscopic resections
Although ESD is a well-accepted minimally invasive procedure, the procedure requires high technical skills on the part of the operator, is time consuming, and is associated with a high rate of procedural related complications. The major problem lies in the lack of a suitable endoscopic platform and instruments for its performance. Conventional endoscopes were never designed to support the performance of intricate procedures such as ESD. Operation of current endoscopy system suffers severe lack of dexterity. Precision in maneuvers is very difficult to achieve, resulting sometimes in inadvertent incisions leading to bleeding, and even perforation of the gastrointestinal wall. As all current standard endoscopic resections are deployed on a single axis in line with the endoscope, off-axis motions such as the triangulation of surgical instruments are rendered almost impossible.
Furthermore, due to the sheer length of the endoscope, the force transmission from the operator is diminished by time it reaches the target. This results in insufficient force for effectual triangulation, counter-traction and dissection of the tissue (58). With CO2 insufflations manually controlled through the endoscope, continuous maintenance of optimal internal pressure to maintain luminal space and full view of surgical field is not easy. Thus, ESD remains a procedure only performed by those highly skilled in performing intricate endoscopic interventions.
Innovations in ESD instrumentations and future possibilities
In recent years, several innovations providing solutions to easier and safer performance of ESD have emerged, with a few having been commercialized since. Most of these innovations are in the form of auxiliary devices designed to overcome specific technical limitations of current ESD instrumentation, although a few are complete modified therapeutic systems enhanced and/or redesigned to support not only the performance of endoscopic procedures.
Auxiliary endoscopic devices developed thus far address only specific technical shortcomings of currently used therapeutic endoscopic systems. Most notably, the Japanese endoscope manufacturer, Olympus, has delivered the Endolifter®, a dedicated retraction device designed specifically to support performance of ESD (59). Endolifter® makes possible simultaneous grasping, retracting and lifting of the mucosa during ESD, resulting in better visualization of the cutting line in the submucosal tissue layer. Other innovations providing similar benefits include the most recent Endo-Dissector, a German prototype instrument designed specifically for ESD (60), and the Maryland dissector made in the USA (61). Other devices designed to facilitate adequate exposure of the submucosal layer during ESD include a grasping-type scissors forceps (GSF) to grasp and lift the submucosa (62), a rubber strip-based traction device (S-O clip), which when applied to the colon wall enables traction on the edge of the lesion (63), an Impact Shooter (TOP Corp, Japan), which when deployed with the usual therapeutic endoscope provided a two-point fixed ESD system that allows expansion of the mucosal dissection surface to ensure a sufficient visual field throughout the ESD procedure (64), and an externally deployed magnetic controlling device to facilitate magnetic anchor-guided ESD (MAG-ESD) through mucosal lifting for gastric submucosal dissection (65).
More advanced systems include multi-tasking endoscopic platforms such as the EndoSAMURAI (Olympus, Japan) (66), Direct Drive Endoscopic system (DDES) (Boston Scientific, Massachusetts, USA) (67), and the TransPort™ Multi-lumen Operating Platform (USGI medical, California, USA) (68). The EndoSAMURAI is designed with two additional independent flexible channels besides the usual working channel to allow convenient independent deployment of interchangeable surgical instruments.
Although all the aforementioned innovations are significant improvements over the current endoscopy system, these new platforms still fall short of providing a complete solution to the technical problems faced by therapeutic endoscopists today. The solution in sight probably lies in robotics. In a first attempt of its kind, Ho et al. had designed a novel robotic-enhanced Master and Slave Transluminal Endoscopic Robot (MASTER) (69). Unlike the other contemporary innovations, MASTER uses robotic technology to facilitate full instrumental mobility and completely separates control of end-effector’s motion from that of endoscopic movement. Surgical tasks are instead independently and intuitively executed via a human-machine interface, by a second operator. This enables bimanual coordination of interchangeable effecter instruments to facilitate actions such as retraction/exposure, traction/counter traction, approximation and dissection of tissue (70,71). The MASTER is currently being further developed with an array of auxiliary devices and swappable end-effectors to support both endoluminal and transluminal endoscopic surgery. A dedicated suturing system for safe luminal closure with MASTER is in early stage of development. In terms of providing a safe surgical environment within the abdomen, the use of MASTER with steady pressure automatically controlled endoscopy (SPACE) (72), a novel platform for flexible gastrointestinal endoscopy developed by Endeavor for Next Generation of Interventional Endoscopy (ENGINE) in Japan is currently being explored. They increase control of surgical effectors manipulating the target tissue and enhance performance in complex surgical tasks.
Very recently, the use of the laparoscopic and endoscopic cooperative surgery (LECS) (73) procedure is indicated for EGC that would be difficult to treat with ESD, including large lesions located at the greater curvature of the gastric body or fornix, or for lesions with strong ulcerative changes (74). As same concept, to prevent the risk of cancer cells seeding during open gastrectomy, non-exposure gastric wall full-thickness resection techniques such as ‘‘CLEAN-NET’’ (75) or ‘‘NEWS’’ (76) have been developed. CLEAN-NET is a technique for the full-thickness resection of the stomach wall using only laparoscopy and then using endoscopy to confirm the dissected line, whereas NEWS is a technique that uses endoscopy to assist with the laparoscopic approach. However, the mucosal layer shifts significantly from the seromuscle layer during surgery, so that the muscle layer and sero-muscle layer may be incorrectly dissected using CLEAN-NET and NEWS. These can result in the inappropriate resection of the stomach wall.
LECS is safe and feasible, with a reasonable surgical time. If the sentinel lymph node concept is established in the surgical treatment for gastric cancer, then the indications for LECS for EGC could be expanded in the future, which could result in increasingly successful gastric cancer treatment.
Acknowledgements
Dr. Gotoda is the lecturer for Olympus.
Disclosure: The authors declare no conflict of interest.
References
- Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg 1950;38:175-82. [PubMed]
- Sano T, Sasako M, Kinoshita T, et al. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer 1993;72:3174-8. [PubMed]
- Sasako M, Kinoshita T, Maruyama K. Prognosis of early gastric cancer. Stomach Intest 1993;28:139-46.
- Ohta H, Noguchi Y, Takagi K, et al. Early gastric carcinoma with special reference to macroscopic classification. Cancer 1987;60:1099-106. [PubMed]
- Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg 1997;84:1567-71. [PubMed]
- Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg 1992;79:241-4. [PubMed]
- Ludwig K, Klautke G, Bernhard J, et al. Minimally invasive and local treatment for mucosal early gastric cancer. Surg Endosc 2005;19:1362-6. [PubMed]
- Hull MJ, Mino-Kenudson M, Nishioka NS, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol 2006;30:114-8. [PubMed]
- Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc 2002;55:390-6. [PubMed]
- Yanai H, Matsubara Y, Kawano T, et al. Clinical impact of strip biopsy for early gastric cancer. Gastrointest Endosc 2004;60:771-7. [PubMed]
- Farrell JJ, Lauwers GY, Brugge WR. Endoscopic mucosal resection using a cap-fitted endoscope improves tissue resection and pathology interpretation: an animal study. Gastric Cancer 2006;9:3-8. [PubMed]
- Gotoda T, Sasako M, Ono H, et al. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg 2001;88:444-9. [PubMed]
- Korenaga D, Haraguchi M, Tsujitani S, et al. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg 1986;73:431-3. [PubMed]
- Ell C, May A, Gossner L, et al. Endoscopic mucosectomy of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670-7. [PubMed]
- Tanabe S, Koizumi W, Mitomi H, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc 2002;56:708-13. [PubMed]
- Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc 2007;66:693-700. [PubMed]
- Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-9. [PubMed]
- Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331-6. [PubMed]
- Yokoi C, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc 2006;64:212-8. [PubMed]
- Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007;10:1-11. [PubMed]
- Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 2006;41:929-42. [PubMed]
- Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [PubMed]
- Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005;23:4490-8. [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-225. [PubMed]
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009;12:148-52. [PubMed]
- Isomoto H, Ohnita K, Yamaguchi N, et al. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur J Gastroenterol Hepatol 2010;22:311-7. [PubMed]
- Kusano C, Iwasaki M, Kaltenbach T, et al. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol 2011;106:1064-9. [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [PubMed]
- Leung JW, Chan FK, Sung JJ, et al. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factors and the role of epinephrine injection. Gastrointest Endosc 1995;42:550-4. [PubMed]
- Uedo N, Iishi H, Tatsuta M, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer 2006;9:88-92. [PubMed]
- Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 2011;73:942-8. [PubMed]
- Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868-71. [PubMed]
- Nagano H, Ohyama S, Fukunaga T, et al. Two rare cases of node-positive differentiated gastric cancer despite their infiltration to sm1, their small size, and lack of lymphatic invasion into the submucosal layer. Gastric Cancer 2008;11:53-7; discussion 57-8. [PubMed]
- Oya H, Gotoda T, Kinjo T, et al. A case of lymph node metastasis following a curative endoscopic submucosal dissection of an early gastric cancer. Gastric Cancer 2012;15:221-5. [PubMed]
- Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Digest Endosc 2005;17:54-8.
- Fujishiro M, Ono H, Gotoda T, et al. Usefulness of Maalox for detection of the precise bleeding points and confirmation of hemostasis on gastrointestinal hemorrhage. Endoscopy 2001;33:196. [PubMed]
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy 2008;40:179-83. [PubMed]
- Okano A, Hajiro K, Takakuwa H, et al. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc 2003;57:687-90. [PubMed]
- Minami S, Gotoda T, Ono H, et al. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 2006;63:596-601. [PubMed]
- Hirasawa R, Iishi H, Tatsuta M, et al. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc 1997;46:507-13. [PubMed]
- Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006;63:243-9. [PubMed]
- Ikehara H, Gotoda T, Ono H, et al. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg 2007;94:992-5. [PubMed]
- Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, et al. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc 2009;69:350-5. [PubMed]
- Catalano F, Trecca A, Rodella L, et al. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc 2009;23:1581-6. [PubMed]
- Coda S, Trentino P, Antonellis F, et al. A Western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer 2010;13:258-63. [PubMed]
- Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc 2012;75:276-86. [PubMed]
- Kakushima N, Fujishiro M, Kodashima S, et al. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy 2006;38:991-5. [PubMed]
- Oda I, Odagaki T, Suzuki H, et al. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 2012;24:129-32. [PubMed]
- Goda K, Fujishiro M, Hirasawa K, et al. How to teach and learn endoscopic submucosal dissection for upper gastrointestinal neoplasm in Japan. Dig Endosc 2012;24:136-42. [PubMed]
- Ohnita K, Isomoto H, Yamaguchi N, et al. Factors related to the curability of early gastric cancer with endoscopic submucosal dissection. Surg Endosc 2009;23:2713-9. [PubMed]
- Hirasawa K, Kokawa A, Oka H, et al. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1268-75. [PubMed]
- Yamamoto S, Uedo N, Ishihara R, et al. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy 2009;41:923-8. [PubMed]
- Parra-Blanco A, Gonzalez N, Arnau MR. Ex vivo and in vivo models for endoscopic submucosal dissection training. Clin Endosc 2012;45:350-7. [PubMed]
- Parra-Blanco A, Arnau MR, Nicolás-Pérez D, et al. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol 2010;16:2895-900. [PubMed]
- Berr F, Ponchon T, Neureiter D, et al. Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc 2011;23:281-9. [PubMed]
- Gotoda T, Friedland S, Hamanaka H, et al. A learning curve for advanced endoscopic resection. Gastrointest Endosc 2005;62:866-7. [PubMed]
- Draganov PV, Gotoda T, Chavalitdhamrong D, et al. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013;78:677-88. [PubMed]
- Kondo H, Gotoda T, Ono H, et al. Percutaneous traction-assisted EMR by using an insulation-tipped electrosurgical knife for early stage gastric cancer. Gastrointest Endosc 2004;59:284-8. [PubMed]
- Teoh AY, Chiu PW, Hon SF, et al. Ex vivo comparative study using the Endolifter® as a traction device for enhancing submucosal visualization during endoscopic submucosal dissection. Surg Endosc 2013;27:1422-7. [PubMed]
- Meining A, Schneider A, Roppenecker D, et al. A new instrument for endoscopic submucosal dissection (with videos). Gastrointest Endosc 2013;77:654-7. [PubMed]
- von Renteln D, Dulai PS, Pohl H, et al. Endoscopic submucosal dissection with a flexible Maryland dissector: randomized comparison of mesna and saline solution for submucosal injection (with videos). Gastrointest Endosc 2011;74:906-11. [PubMed]
- Akahoshi K, Honda K, Motomura Y, et al. Endoscopic submucosal dissection using a grasping-type scissors forceps for early gastric cancers and adenomas. Dig Endosc 2011;23:24-9. [PubMed]
- Sakamoto N, Osada T, Shibuya T, et al. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc 2009;69:1370-4. [PubMed]
- Motohashi O. Two-point fixed endoscopic submucosal dissection in rectal tumor (with video). Gastrointest Endosc 2011;74:1132-6. [PubMed]
- Gotoda T, Oda I, Tamakawa K, et al. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc 2009;69:10-5. [PubMed]
- Spaun GO, Zheng B, Swanström LL. A multitasking platform for natural orifice translumenal endoscopic surgery (NOTES): a benchtop comparison of a new device for flexible endoscopic surgery and a standard dual-channel endoscope. Surg Endosc 2009;23:2720-7. [PubMed]
- Thompson CC, Ryou M, Soper NJ, et al. Evaluation of a manually driven, multitasking platform for complex endoluminal and natural orifice transluminal endoscopic surgery applications (with video). Gastrointest Endosc 2009;70:121-5. [PubMed]
- Clayman RV, Box GN, Abraham JB, et al. Rapid communication: transvaginal single-port NOTES nephrectomy: initial laboratory experience. J Endourol 2007;21:640-4. [PubMed]
- Ho KY, Phee SJ, Shabbir A, et al. Endoscopic submucosal dissection of gastric lesions by using a Master and Slave Transluminal Endoscopic Robot (MASTER). Gastrointest Endosc 2010;72:593-9. [PubMed]
- Wang Z, Phee SJ, Lomanto D, et al. Endoscopic submucosal dissection of gastric lesions by using a master and slave transluminal endoscopic robot: an animal survival study. Endoscopy 2012;44:690-4. [PubMed]
- Phee SJ, Reddy N, Chiu PW, et al. Robot-assisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin Gastroenterol Hepatol 2012;10:1117-21. [PubMed]
- Nakajima K, Moon JH, Tsutsui S, et al. Esophageal submucosal dissection under steady pressure automatically controlled endoscopy (SPACE): a randomized preclinical trial. Endoscopy 2012;44:1139-48. [PubMed]
- Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. [PubMed]
- Nunobe S, Hiki N, Gotoda T, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer 2012;15:338-42. [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. [PubMed]
- Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer 2011;14:183-7. [PubMed]


