Endoscopic submucosal dissection for colorectal neoplasms
Introduction
Colorectal superficial neoplasms, especially those limited to the intramucosal, comprise a clinically good indication for endoscopic treatment. Endoscopic mucosal resection (EMR) using an electric snare is widely used as an established safe and easy procedure. However, lesions exceeding 20 mm in diameter must often be removed in piecemeal fashion, and the rate of recurrence for such tumors is higher than that for those removed via en bloc resection (1-4). Most residual or recurrent lesions following piecemeal EMR are considered due to residual tissue in the outer and inner resection margins; thus, the incidence rate of such recurrent lesions is expected to increase as the number of resected specimens increases (1). The local luminal recurrence rate after piecemeal EMR has been reported to be approximately 10-20%, and salvage endoscopic treatment is often difficult to perform by conventional EMR due to severe fibrosis following the original procedure (2-5).
Endoscopic resection plays two important roles in gastrointestinal surgery, i.e., achieving curative resection and allowing accurate histological evaluation of the lesions. Lesions measuring ≥10 mm carry the possibility of malignant potential; therefore, such tumors should be resected en bloc to avoid residual and/or recurrent lesions.
Endoscopic submucosal dissection (ESD) is a state-of-the art technique for the treatment of large colorectal neoplasms that makes it possible to achieve en bloc resection regardless of lesion size (6-13). This review describes the details of ESD for colorectal neoplasms along with a description of some of the devices used for this procedure.
Indications for ESD
In Japan, colorectal ESD has been covered under health insurance since 2012. Before this date, colorectal ESD was allowed to be performed only at a limited number of advanced medical institutions approved by the Japanese Ministry of Health, Labor and Welfare in 2009; accordingly, “The Colon ESD Standardization Implementation Working Group”, a subordinate organization of the “Gastroenterological Endoscopy Promotion Liaison Conference”, proposed a draft of “Criteria of Indications for Colorectal ESD”. Essentially, ESD is indicated when lesions need to be resected en bloc in order to evaluate their histological features and when they are difficult to resect by the conventional EMR technique. Namely, cancerous lesions that are potentially invasive into the submucosal layer should be treated by ESD. In such lesions, the lesion size and morphology are considered very important factors; for example, laterally spreading tumors (LST) of the granular, nodular mixed type (LST-G-MIX) measuring ≥30 mm as well as LST of the non-granular typed measuring ≥20 mm has been considered to harbor some risk of an invasive component. Second, lesions that would be very difficult to resect via conventional EMR from the technical viewpoint are also considered as an indication for ESD, including lesions showing non-lifting after submucosal injection, local recurrent lesions following previous treatment, and relatively large protruded type lesions, except pedunculated polyps. In general, in large neoplastic lesions measuring ≥20 mm, it is technically difficult to achieve en bloc resection by conventional EMR, and piecemeal EMR is typically applied. Undoubtedly, piecemeal EMR is an important method for lesions that carry minimal potential for submucosal invasion, such as intramucosal neoplasms; however, it is crucial to recognize an important demerit of piecemeal EMR, i.e., higher risk of local recurrence. We have previously reported that the removal of five or more specimens from a single patient is an independent risk factor of local recurrence following piecemeal EMR (1). Therefore, careful surveillance colonoscopy is needed after multiple piecemeal EMR. Considering the risk and occurrence of invasive recurrence in piecemeal EMR-treated patients, it is advisable to avoid such multiple resections and explore alternative treatment strategies. Moreover, we reported three cases required surgery after piecemeal EMR including two cases of invasive recurrence (14). Hence, we should consider for ESD or surgery as first treatment for lesions in which precise pathological evaluation would be disturbed by multiple piecemeal EMR (15).
Diagnosis
Before initiating therapy for a colorectal lesion, it is essential to accurately evaluate the depth of invasion by the lesion. Magnifying observation techniques, including chromoendoscopy and narrow-band imaging (NBI), have been recognized as high-precision methods for this purpose (16,17). In NBI, avascular or loose vascular findings are considered as a key sign indicative of submucosal deep invasive cancer. However, the NBI system is a relatively new diagnostic method with an unknown learning curve and several different classifications even in a single country such as Japan. On the other hand, pit pattern analysis using crystal violet staining has been standardized due to its longer availability and one-to-one comparison between endoscopic and pathological findings. We consider that pit pattern analysis is the most reliable method for the prediction of depth invasion. In pit pattern analysis, a non-invasive pattern and Type V pit(s) with a clear demarcated area should be confirmed in each lesion, indicating that the lesion is suitable for EMR or ESD, with the estimated invasion depth being less than submucosal invasive cancer (18). No biopsies are performed in patients undergoing ESD because they can cause fibrosis and may interfere with submucosal lifting.
Strategy
Preparation
One of the important key elements of safe ESD is a well-cleansed colon in order to avoid adverse events such as bacterial peritonitis following iatrogenic colonic wall perforation. In our hospital, patients typically receive 3-4 L of polyethylene glycol over a 4-h period in the morning prior to ESD. Further, 1 g of cefmetazole in 100 mL of saline solution is administered 20-30 min before the ESD procedure.
Sedation
An anti-peristaltic agent (10 mg of scopolamine butylbromide or 0.5 mg of glucagon) is injected intravenously as mandatory medication, and a sedative (2-3 mg of midazolam) and an analgesic (15 mg of pentazocine) are administered intravenously as needed throughout the procedure. It is essential to maintain conscious sedation during the procedure since patients are sometimes required to change their position, based on the direction of gravity that allows the dissected part of the lesion to hang downward for improved recognition of the submucosal layer.
Treatment devices
In this section, we have described the devices typically used in our institution. The water jet endoscope (PCF-Q260JI and GIF-Q260J; Olympus Medical System Co., Tokyo, Japan) is used for ESD. When it would be difficult to handle the scope just as operator intended during the ESD procedure due to the location of the lesion or paradoxical movements, the double-balloon colonoscope (EC-450BI, Fujifilm, Japan) is one of the options available for the precise control of the endoscope (19).
The ball-tip bipolar needle knife with water jet function (Jet B knife; XEMEX Co., Tokyo, Japan) is used for mucosal incision and submucosal dissection in the initial part of the procedure (Figure 1A). A notable characteristic of this device is that it uses the bipolar current system, which minimizes damage to deeper tissues and reduces the risk of perforation (20,21). Then, the insulation-tipped electrosurgical knife (IT knife nano, KD-612Q; Olympus Optical Co., Tokyo, Japan), wherein the insulation tip is smaller and the short blade is designed as a small disk to reduce the burning effect on the muscular layer, is usually used to shorten the procedure time (Figure 1B) (22).

For distal attachment, we use the ST hood short-type (DH-28GR and 29CR; Fujifilm Medical Co., Tokyo, Japan), which renders it easy to broaden the operator’s visual field and to dissect the submucosal layer due to its characteristic tapering shape (Figure 1C).
Electrosurgical current generator
In our institution, we mainly use the ERBE VIO 300 D (Erbe, Tubingen, Germany). Its output settings for ESD procedures are described in Table 1.

Full table
Submucosal injection
The maintenance of adequate submucosal elevation by injection is a key component controlling the success and failure of ESD procedures. Submucosal injection solutions that provide a longer duration of submucosal elevation are therefore preferable. Accordingly, we use two solutions in our hospital, Glyceol (10% glycerin and 5% fructose; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) mixed with small amounts of indigocarmine and epinephrine and 0.4% sodium hyaluronate solution (MucoUp; Seikagaku Corp, Tokyo, Japan) (23). In practice, a small amount of Glyceol is first injected into the submucosal layer to confirm appropriate submucosal layer elevation; subsequently, MucoUp is injected into the properly elevated submucosal layer; and finally, a small amount of Glyceol is injected again to flush any residual amount of MucoUp (24).
Carbon dioxide (CO2) insufflation
CO2 gas should be used for insufflation for the colonic lumen, since its effectiveness has been proven (25,26). CO2 insufflation can reduce the risk of pneumoperitoneum in case of perforation as well as the occurrence of abdominal complaints before and/or after treatment.
Technique of ESD
Here, we describe the important points of the ESD technique, as followed in our institution.
- The process is initiated in the retroflex view since it can stabilize the handling of the endoscope better than the forward view. Then, after achieving sufficient submucosal elevation by injection, the initial mucosal incision is made with the Jet B knife from the distal side of the lesion.
When it is difficult to obtain a retroflex view, the tunneling method reported by Yamamoto is useful (27). In this method, mucosal incision and trimming of the mucosa are initiated from the distal side of the lesion until the final tissue segment is reached; then, mucosal incision and submucosal dissection are performed from the proximal side of the lesion. - It is usually difficult to insert the tip of the endoscope into the submucosal layer just after the initial mucosal incision; trimming of the mucosa is performed in such cases. Since the dissection space is not adequate to continue submucosal dissection during trimming, the submucosal layer near the mucosal layer is carefully cut gently.
- After securing the submucosal layer in the visual field, submucosal dissection is continued with the same Jet B-knife. One of the merits of ESD includes the good recognition of structures in the submucosal layer, e.g., vessels, fibrosis, etc.; it is therefore possible to prevent bleeding by performing pre-coagulation of the involved vessels. For thin-walled vessels, pre-coagulation is performed using cutting devices. However, for thick-walled or pulsatile vessels, coagulation forceps should be applied. In our institution, Hemostat-Y forceps (H-S2518; Pentax Co., Tokyo, Japan) are used in the bipolar mode (25 W) to control visible bleeding and minimize the risk of any burning effect on the muscle layer (Figure 1D).
Moreover, in ESD, it is also possible to adjust the cutting line during submucosal dissection. In adenomatous lesions, the cutting line can be set near the mucosal layer to reduce the risk of perforation. On the other hand, for lesions that are potentially of the submucosal invasive cancer type, wherein it is necessary to achieve R0 resection, the cutting line should be set in the deeper tissues, i.e., near the muscularispropria, despite the higher risk of perforation. - After obtaining an adequate visual field as explained above, the IT knife nano is used for continuous dissection. This knife, which is a “blade” type knife, has a longer usable section that can shorten the procedure time as compared with other “needle” type knives. Throughout the procedure, repeated submucosal injections should be administered to maintain good submucosal elevation.
- After colorectal ESD is completed, a routine colonoscopic review to detect any possible perforation or exposed vessels is conducted and minimum coagulation is performed using the hemostat-Y forceps on non-bleeding visible vessels to prevent postoperative bleeding.
Outcomes
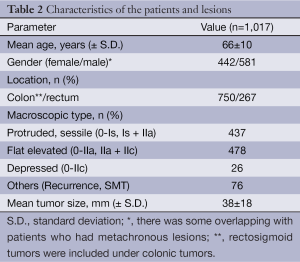
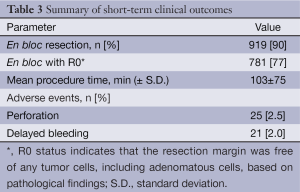
The short-term outcomes of 1,017 colorectal ESD procedures performed in our institution are described in Tables 2,3. The mean lesion size was 38 mm in diameter. En bloc resection was achieved in 90% cases (919/1,017), with a perforation rate of 2.5% (25/1,017) and delayed bleeding rate of 2.0% (21/1,017). Delayed bleeding was defined as evident hematochezia or melena developed after the completion of the procedure and requiring colonoscopy again. Overall, two of the perforation cases required emergency surgery, while the others were managed conservatively, with nil by mouth, antibacterial therapy and insertion of decompression tube in some cases. Most cases of iatrogenic perforation usually involve very small perforations that can be closed by endoscopic clip placement. Among the patients who experiences delayed bleeding, none of them required blood transfusions.
Full table
Full table
Future outlook
Various treatment materials have been developed and applied in ESD subsequent to the introduction of this technique. However, there is no exact standardized procedure for ESD, and it is important to continue efforts toward improving the safety and technical ease of the procedure.
In maintaining good submucosal elevation, for example, the Endolifter, S-O clip (28), and clip-flap (29) method make it possible to improve visualization and stability in the cutting area when used as traction devices. For iatrogenic perforation during ESD, endoscopic closure using endoclip(s) is a popular technique. Recently, we reported the efficacy of a novel technique for the closure of iatrogenic perforations via a small mucosal incision around the perforation, providing a better grip for the clip (30). This technical alteration improved the probability of complete closure. Further, the over-the-scope clip (OTSC) using a twin grasper (Ovesco Endoscopy GmbH, Tuebingen, Germany) is an interesting device that enables safe closure as a full-thickness suturing device (31). These types of refinements in the ESD technique and equipment are expected to increase the safety of the ESD procedure and lead to its worldwide adoption in the near future.
Conclusions
This review has outlined the details of colorectal ESD. Notably, this technique is a reliable method for achieving en bloc resection of relatively large colorectal superficial neoplasms, with superior curability and allowing accurate pathological evaluation as compared to piecemeal EMR. Moreover, colorectal ESD has succeeded in reducing over surgery of mucosal carcinomas and improving the overall quality of life in patients with lower rectal lesions. However, the technical difficulty of ESD and its associated complications, such as iatrogenic perforation, have hampered its worldwide adoption. We expect this to change in the future with refinements of available treatment devices that improve the safety and technical ease of ESD.
Acknowledgements
Disclosure: Our retrospective and prospective studies cited in this review were partially supported by the National Cancer Center Research and Development Fund (23-A-19 and 23-B-17).
References
- Sakamoto T, Matsuda T, Otake Y, et al. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol 2012;47:635-40. [PubMed]
- Soetikno RM, Inoue H, Chang KJ. Endoscopic mucosal resection. Current concepts. Gastrointest Endosc Clin N Am 2000;10:595-617. [PubMed]
- Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001;54:62-6. [PubMed]
- Tamura S, Nakajo K, Yokoyama Y, et al. Evaluation of endoscopic mucosal resection for laterally spreading rectal tumors. Endoscopy 2004;36:306-12. [PubMed]
- Sakamoto T, Saito Y, Matsuda T, et al. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc 2011;25:255-60. [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 2007;5:678-83. [PubMed]
- Nishiyama H, Isomoto H, Yamaguchi N, et al. Endoscopic submucosal dissection for laterally spreading tumours of the colorectum in 200 consecutive cases. Surg Endosc 2010;24:2881-7. [PubMed]
- Saito Y, Sakamoto T, Fukunaga S, et al. Endoscopic submucosal dissection (ESD) for colorectal tumors. Dig Endosc 2009;21:S7-12. [PubMed]
- Niimi K, Fujishiro M, Kodashima S, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2010;42:723-9. [PubMed]
- Yoshida N, Naito Y, Kugai M, et al. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol 2010;16:4180-6. [PubMed]
- Toyonaga T, Man-i M, Chinzei R, et al. Endoscopic treatment for early stage colorectal tumors: the comparison between EMR with small incision, simplified ESD, and ESD using the standard flush knife and the ball tipped flush knife. Acta Chir Iugosl 2010;57:41-6. [PubMed]
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013;27:3262-70. [PubMed]
- Saito Y, Kawano H, Takeuchi Y, et al. Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc 2012;24:67-72. [PubMed]
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010;24:343-52. [PubMed]
- Kiriyama S, Saito Y, Yamamoto S, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy 2012;44:1024-30. [PubMed]
- Sakamoto T, Saito Y, Nakajima T, et al. Comparison of magnifying chromoendoscopy and narrow-band imaging in estimation of early colorectal cancer invasion depth: a pilot study. Dig Endosc 2011;23:118-23. [PubMed]
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33. [PubMed]
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008;103:2700-6. [PubMed]
- Ohya T, Ohata K, Sumiyama K, et al. Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol 2009;15:6086-90. [PubMed]
- Sano Y, Fu KI, Saito Y, et al. A newly developed bipolar-current needle-knife for endoscopic submucosal dissection of large colorectal tumors. Endoscopy 2006;38:E95. [PubMed]
- Nonaka S, Saito Y, Fukunaga S, et al. Impact of endoscopic submucosal dissection knife on risk of perforation with an animal model-monopolar needle knife and with a bipolar needle knife. Dig Endosc 2012;24:381. [PubMed]
- Hotta K, Yamaguchi Y, Saito Y, et al. Current opinions for endoscopic submucosal dissection for colorectal tumors from our experiences: indications, technical aspects and complications. Dig Endosc 2012;24:110-6. [PubMed]
- Uraoka T, Fujii T, Saito Y, et al. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc 2005;61:736-40. [PubMed]
- Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 2003;35:690-4. [PubMed]
- Saito Y, Uraoka T, Matsuda T, et al. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc 2007;65:537-42. [PubMed]
- Kikuchi T, Fu KI, Saito Y, et al. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: a prospective study. Surg Endosc 2010;24:2231-5. [PubMed]
- Mönkemüller K, Wilcox CM, Muñoz-Navas M. eds. Interventional and Therapeutic Gastrointestinal Endoscopy. Frontiers of Gastrointestinal Research, 2010;27.
- Sakamoto N, Osada T, Shibuya T, et al. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc 2009;69:1370-4. [PubMed]
- Yamamoto K, Hayashi S, Nakabori T, et al. Endoscopic submucosal dissection using endoclips to assist in mucosal flap formation (novel technique: “clip flap method”). Endoscopy 2012;44:E334-5. [PubMed]
- Otake Y, Saito Y, Sakamoto T, et al. New closure technique for large mucosal defects after endoscopic submucosal dissection of colorectal tumors (with video). Gastrointest Endosc 2012;75:663-7. [PubMed]
- Nishiyama N, Mori H, Kobara H, et al. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol 2013;19:2752-60. [PubMed]


