Cell sheets engineering for esophageal regenerative medicine
Tissue engineering using cell sheet
Regeneration of tissues and organs offers an innovative approach to the treatment of injury and disease with substituted artificial organs and organ transplantations. The term ‘‘tissue engineering’’ was first utilized in the late 1980s, the use of cultured cells as a therapeutic modality was initiated by Howard Green and associates, who transplanted cultured sheets of autologous epidermis to patients resulting from severe burns, giant congenital nevi, and skin ulcers. The key technology is the use of biodegradable polymer scaffolds, preformed in the target tissue shape, for seeding cells, as demonstrated in the well-publicized reconstruction of cartilage tissues for the growth of human ears on mouse back. Since the mid-1990s, significant progress from basic science has resulted in clinical applications of tissue engineering for the replacement of a variety of tissues and organs. An innovative approach to tissue engineering using a thermo-responsive culture surface has been developed from 1990s (1). Poly (N-isopropylacrylamide) (PIPAAm) exhibits a lower critical solution temperature (LCST) in aqueous media at the vicinity of 32 °C. While they hydrate and form an expanded structure in an aqueous media below the LCST, they dehydrate and form a compact structure above the LCST. Such a conformational change in response to temperature has been extensively used to modulate the physicochemical properties of polymeric thin surfaces. At 37 °C, PIPAAm-grafted surface is slightly hydrophobic, allowing cells to proliferate under normal conditions and become a confluent cell sheet, which is regularly found on usual tissue culture polystyrene. A decrease in temperature lower than 32 °C, however, results in the hydration of the polymer surface, giving the spontaneous detachment of the cells as a monolithic tissue-like cell sheet for less than 1 h without any enzymes such as trypsin. Since PIPAAm is covalently immobilized onto the culture surfaces, PIPAAm remains bound to the surfaces even after cell sheet detachment, realizing the non-invasive harvest of cultured cells as an intact cell sheet having deposited ECM. This technology allows us to transplant cell sheets to host tissues without using biodegradable scaffolds. PIPAAm thickness on the order of nanometers is necessary for expressing such interesting properties as temperature-controlled cell attachment or detachment. For example, a PIPAAm layer of approximately 20 nm is optimal for cell adhesion and detachment properties in response to temperature change for a PIPAAm-modified tissue culture polystyrene system. Since the PIPAAm-grafted surfaces facilitate spontaneous cell detachment, the use of conventional proteolytic enzymes such as dispase, trypsin, and collagenase, can be avoided. With noninvasive cell harvest, cell-to-cell junction and ECM proteins can therefore be maintained (2) (Figure 1).
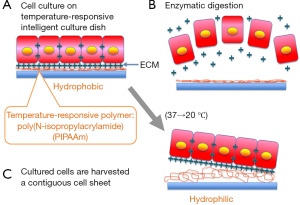
Numerous cell types including epidermal keratinocytes (3), vascular endothelial cells (4), renal epithelial cells, periodontal ligaments (5,6), and cardiomyocytes (7,8) have shown the maintenance of differentiated functions after low-temperature cell sheet harvest, due to the preservation of cell surface proteins, such as growth factor receptors, ion channels, and cell-to-cell junction proteins. Additionally, due to the presence of deposited ECM that is produced during in vitro incubation, cell sheets can be easily transplanted and attached to the site such as culture dishes and even host tissues. To fabricate thick tissues, cell sheets can be stacked in layers because cell sheets connect each other in a short time. Therefore, cell sheet technology enables us to avoid scaffolds, fixation, or sutures which are needed by conventional tissue engineering approaches using isolated cell injections and scaffold-based technologies, which is the limitation of applicability.
Cell sheet regenerative medicine following endoscopic submucosal dissection (ESD) for esophageal neoplasm
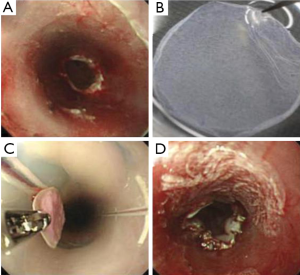
Endoscopic treatments for early esophageal cancer and Barrett’s esophagus with high-grade dysplasia have gained widespread acceptance as minimally invasive therapies (9). In particular, ESD makes it possible to resect superficial cancer en block regardless of size and allows for an accurate histological assessment for diagnosis. However, severe postoperative esophageal stricture is inevitably observed when ESD is performed for widespread superficial neoplasms that remove a large area of mucosa, i.e., >75% of the esophageal lumen (10). Severe esophageal stricture after endoscopic treatment is difficult to treat and often requires repeated endoscopic balloon dilations (10,11) or a temporary stent (12). It has been reported that fibrosis of the submucosa and atrophy of the muscularis contribute to esophageal stricture following endoscopic treatment (13). Therefore, the prevention of esophageal stricture requires intervention aimed at reducing mucosal defects, calming inflammation, and preventing excess fibrosis. For solving the demand, our laboratory has developed a method combining ESD with the endoscopic transplantation of autologous oral mucosal epithelial cell sheets (14) (Figure 2). The purpose of our approach is to cover the surface of mucosal defects to promote re-epithelialization and to reconstruct the esophageal luminal surface by using cell sheets. Results showed the effectiveness of a novel combined endoscopic approach for the potential treatment of esophageal cancers that can effectively enhance wound healing and possibly prevent postoperative esophageal stenosis. In addition, we reported using fabricated autologous skin epidermal cell sheet, as another epithelial cell source, prevent severe stricture following full-circumferential ESD in a porcine model (15) (Figure 3). These transplantable epithelial cell sheets are fabricated on temperature-responsive culture surfaces, which allow noninvasive harvesting of the cell sheets simply by reducing the temperature below 32 °C; the fabricated cell sheets retain all of the cell membrane proteins and extracellular matrices deposited during culture. Therefore, these cell sheets can easily adhere to and integrate into transplanted tissue sites within a short period. Additionally, the placement of cell sheets into the mucosal defect does not require sutures or other adhesives. Furthermore, several studies revealed epithelial cell sheets can be fabricated with temperature-responsive culture inserts without feeder layers (16), which can exclude xenogeneic factors for animal-free cell transplantation.
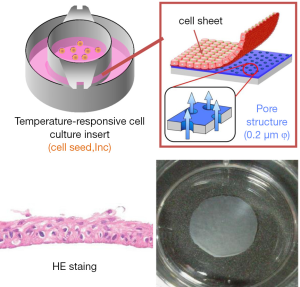
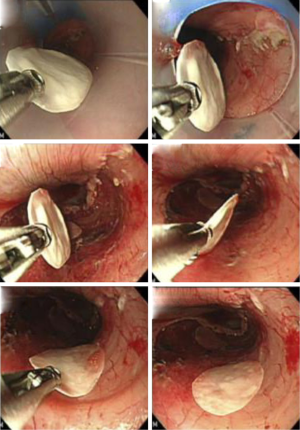
Based on these animal studies, human autologous oral mucosal epithelial cell sheets are fabricated with the UpCell-Insert technology (17). The first clinical study was esophageal regeneration using autologous oral mucosal cell sheets following a large-size removal ESD for superficial esophageal neoplasms and the results were published in 2012 (18). The preliminary results showed that early re-epithelialization of the ulcer site and the results suggested its efficacy for preventing stricture in case of circumferential ESD (Figure 4). Currently, we are preparing for a further randomized study to fully assess the potential benefits of regenerative medicine using cultured autologous cell sheets therapies.
Conclusions
We described the technology of “Cell Sheet Engineering” and its current applications following endoscopic therapy for esophageal neoplasm. Cell sheet engineering has already been tested in clinical settings for several incurable diseases. We believe that methods that can effectively apply cell sheet engineering will provide new possibilities in the field of regenerative medicine.
Acknowledgements
This work was supported by The High-Tech Research Center Program, Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology ‘Cell Sheet Tissue Engineering Center (CSTEC)’ and the Global COE program, Multidisciplinary Education and Research Center for Regenerative Medicine (MERCREM), from the Ministry of Education, Culture, Sports Science, and Technology (MEXT), Japan.
Disclosure: The following authors disclosed financial relationships relevant to this publication: Dr. Okano, investor in CellSeed. The other authors disclosed no financial relationships relevant to this publication.
References
- Okano T, Yamada N, Sakai H, et al. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res 1993;27:1243-51. [PubMed]
- Kushida A, Yamato M, Konno C, et al. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res 1999;45:355-62. [PubMed]
- Yamato M, Utsumi M, Kushida A, et al. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng 2001;7:473-80. [PubMed]
- Yamato M, Okuhara M, Karikusa F, et al. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J Biomed Mater Res 1999;44:44-52. [PubMed]
- Akizuki T, Oda S, Komaki M, et al. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res 2005;40:245-51. [PubMed]
- Hasegawa M, Yamato M, Kikuchi A, et al. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng 2005;11:469-78. [PubMed]
- Shimizu T, Yamato M, Akutsu T, et al. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res 2002;60:110-7. [PubMed]
- Shimizu T, Yamato M, Kikuchi A, et al. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng 2001;7:141-51. [PubMed]
- Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 1999;50:560-3. [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661-5. [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. [PubMed]
- Rajan E, Gostout C, Feitoza A, et al. Widespread endoscopic mucosal resection of the esophagus with strategies for stricture prevention: a preclinical study. Endoscopy 2005;37:1111-5. [PubMed]
- Honda M, Nakamura T, Hori Y, et al. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy 2010;42:1092-5. [PubMed]
- Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006;55:1704-10. [PubMed]
- Kanai N, Yamato M, Ohki T, et al. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc 2012;76:873-81. [PubMed]
- Murakami D, Yamato M, Nishida K, et al. The effect of micropores in the surface of temperature-responsive culture inserts on the fabrication of transplantable canine oral mucosal epithelial cell sheets. Biomaterials 2006;27:5518-23. [PubMed]
- Takagi R, Yamato M, Murakami D, et al. Preparation of keratinocyte culture medium for the clinical applications of regenerative medicine. J Tissue Eng Regen Med 2011;5:e63-73. [PubMed]
- Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012;143:582-8.e1-2.


