
Infections and risk of end-stage renal disease in patients with nephrotic syndrome: a nationwide population-based case-control study
Introduction
Nephrotic syndrome (NS) is a common renal disorder characterized by the presence of nephrotic range of proteinuria, hypoalbuminemia, and hyperlipidemia (1,2). In Taiwan, membranous nephropathy is the most common cause of NS, whereas focal segmental glomerulosclerosis is the most common in Brazil and Saudi Arabia (3,4).
Infection is one of the most common complications and is a significant cause of morbidity in patients with NS (5,6). Infection may precipitate episodes or induce relapse during remission in patients with NS. Moreover, NS is often associated with occurrence of infection due to relative immunodeficiency compared with healthy people, resulting in unfavorable responsiveness to therapy (7-9). In this study, we hypothesized that infectious incidents can increase the risk of progression to end-stage renal disease (ESRD) in patients with NS. Although infection is one of the most common complications in patients with NS, data on the occurrence of infection and its effect on renal prognosis in patients with NS are limited.
The association of frequency and severity of infection and the subsequent renal outcome are to be defined. In this study, the occurrence of infections and its effect on renal outcomes in patients with NS were retrospectively analyzed. The aim of this nationwide case-control study was to define risk factors for ESRD and infectious complications in patients with NS.
Methods
Data sources
The National Health Insurance, the health care system in Taiwan, provides national data for medical research, quality of services, clinical practice patterns and accessibility to health care programs. In this study, medical claims data from 2000 to 2013 of the National Health Insurance Research Database (NHIRD) were utilized. The NHIRD contains health care data of the insured population of Taiwan that covers >99% of the 23 million residents (10). The Longitudinal Health Insurance Database, a subset of the NHIRD, comprising 1 million beneficiaries randomly sampled from the NHIRD was used for this research. The study protocol was approved by the Research Ethics Committee of Ditmanson Medical Foundation Chia-yi Christian Hospital (CYCH-IRB-106084).
Study design and population
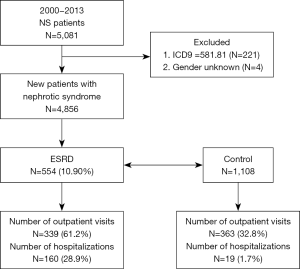
The diagnosis for NS was based on the International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD-9-CM code 581). Inclusion criteria for patients enrolment were as follows: at least three NS diagnoses at the time of outpatient services or one NS diagnosis during an admission. Patients suspected of NS due to specific underlying diseases were excluded (ICD-9-CM code 581.81). Finally, 554 patients with NS with ESRD, and 1,108 random non-ESRD controls matched at a ratio of 1:2 during the same observational period were selected (Figure 1). The population was categorized according to age as young-aged (<40 years), middle-aged (40–65 years), and old-aged (>65 years) groups. The severity of infection was determined based on outpatient visits for acute respiratory infection (ARI) and hospitalization for more severe bacterial infections. ARI included these diagnostic codes: acute nasopharyngitis (ICD-9-CM code 460), acute respiratory tract infections (ICD-9-CM code 464 and 465), bronchiolitis (ICD-9-CM code 466), and bronchitis (ICD-9-CM code 490). Diagnostic codes for bacterial infections included pneumonia (ICD-9-CM code 486), peritonitis (ICD-9-CM code 567), urinary tract infection (UTI; ICD-9-CM code 599.0), cellulitis (ICD-9-CM code 681 and 682), and bacteremia (ICD-9-CM code 038). The number of outpatient visits were classified into four groups, namely 0, 1–5, 6–10, and >10 visits, and the number of hospitalizations were categorized into two groups: 0 and ≥1.
Potential confounders and outcome variable
Potential confounders considered in this study included comorbidities, such as hypertension (ICD-9-CM codes 401–405), diabetes mellitus (ICD-9-CM code 250), congestive heart failure (CHF; ICD-9-CM code 428), hematuria (ICD-9-CM code 599.7) and acute kidney injury (AKI; ICD-9-CM code 584.9). If these diagnostic codes were included in two or more ambulatory claims 6 months before and after the index date, they were considered as comorbidities. Outcome variables were defined as the presence of at least one inpatient claim or two outpatient claims for ESRD (ICD-9-CM code 585) and the issuance of catastrophic illness cards after NS.
Statistical analysis
Demographic data and ESRD risk between the study and control groups were compared using Pearson’s chi-squared test. Conditional logistic regression analyses were performed to calculate the odds ratio (OR) and 95% confidence interval (CI) for having been previously diagnosed with an infection between the two groups. The variables of conditional logistic regression analysis included sex, age, number of outpatient visits, number of hospitalizations, and comorbidities. Further adjustments were performed by stratification of age and sex to evaluate the associations of infection with ESRD. A two-tailed P value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS (version 9.3; SAS institute Inc., Cary, NC, USA).
Results
Clinical characteristics of patients with NS with and without ESRD
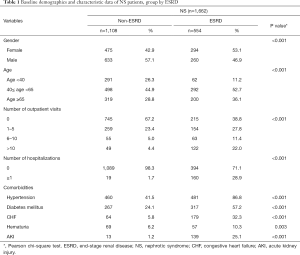
A total of 4,856 patients fulfilled the criteria for the diagnosis of NS between 2000 and 2013; of whom, 554 were identified with ESRD and were included in the case group (Figure 1). Of the 554 patients, 294 (53.1%) were women and 260 (46.9%) were men. The male to female ratio was 1:1.13. The middle-aged group of 40–65 years (52.7%) was predominantly affected (Table 1). A significantly higher proportion of patients with NS with ESRD (22.0%) had more outpatient visits (>10 visits) for ARI compared with patients with NS without ESRD (4.4%). A significantly higher proportion of patients with NS without ESRD (67.2%) had no outpatient visits compared with patients with NS with ESRD (38.8%). Moreover, a significantly higher proportion of patients with NS with ESRD (28.9%) required hospitalization for more severe infections compared with the control group (1.7%). Common causes for hospitalization for infection were UTIs (14.4%), bacteremia (10.3%), and pneumonia (8.8%). Severe infections requiring admissions were significantly associated with a higher risk of ESRD. A trend toward more outpatient visits and inpatient admissions for infections was observed in patients with NS who developed ESRD during the study period.
Full table
Risk of ESRD in patients with NS with and without infections
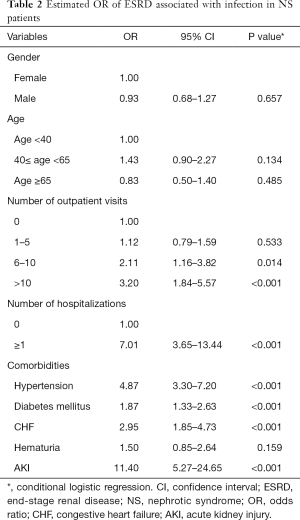
Compared with those with no outpatient visits for infections, the OR associated with ESRD for those with >10 outpatient visits and 6–10 outpatient visits for infections were 3.20 (95% CI, 1.84–5.57) and 2.11 (95% CI, 1.16–3.82), respectively (Table 2). Moreover, compared with those who were not hospitalized for infections, the OR associated with ESRD for those hospitalized admissions was 7.01 (95% CI, 3.65–13.44). Our results suggested differences even after adjustment, with higher rates of infections in patients with NS being associated with subsequent adverse renal outcomes. In addition, patients with hypertension, diabetes mellitus, and CHF tended to have an increased risk of associated ESRD (OR: 4.87, 95% CI, 3.30–7.20, OR: 1.87, 95% CI, 1.33–2.63, and OR: 2.95, 95% CI, 1.85–4.73, respectively). Moreover, patients with AKI had a significantly high risk of associated ESRD (OR: 11.40, 95% CI, 5.27–24.65).
Full table
Association of ESRD with infections according to sex stratification
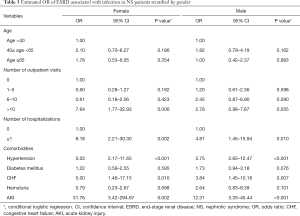
Analysis of data stratified according to sex revealed that the risk of ESRD was significantly higher in female patients with >10 outpatient visits (OR: 7.64, 95% CI, 1.77–32.93) than in male patients (Table 3). Nevertheless, the OR of ESRD was significantly higher in patients with hospitalizations, both in female (OR: 8.18, 95% CI, 2.21–30.30) and male patients (OR: 4.81, 95% CI, 1.45–15.94). This reveals a consistently significant association between high rates of outpatient visits for infections and ESRD in women, although not significant in men. Furthermore, our results emphasize that the risk of ESRD was significantly associated with hospital admissions for infections in both female and male patients. Additionally, patients with NS with hypertension, CHF, or AKI were observed to have a significantly higher risk of ESRD, irrespective of their sex.
Full table
Association of ESRD with infections according to age stratification
Increased OR was found in both middle-aged (40–65 years) and old-aged groups (>65 years) with a higher frequency of outpatient visits (>10 visits), and the risk of ESRD was higher in the old-aged group (OR: 12.58, 95% CI, 2.25–70.22) than in the middle-aged group (OR: 3.82, 95% CI, 1.07–13.64) (Table 4). The OR of ESRD was significantly higher in the middle-aged group (40–65 years) with a history of hospital admissions (OR: 27.41, 95% CI, 2.76–272.08). Moreover, middle-aged patients with hypertension, CHF, and AKI were still observed to have a significantly higher risk of ESRD even after adjustment for confounders. Our results demonstrated a consistently significant association between high rates of outpatient visits for infections and ESRD, and a higher risk was observed, particularly, in elderly patients.
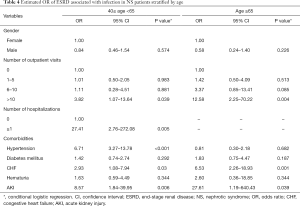
Full table
Discussion
Infections have been identified as a possible cause leading to NS. Immune response secondary to viral infection is a potential trigger of relapse in NS. A close association of respiratory tract infection has been noted with the occurrence, relapse, and aggravation of NS (11,12). In this study, an association between the occurrence of infections and ESRD in patients with NS was further observed. Some patients had mild infection, such as ARI, during the course of NS, and more episodes of ARI (>10 times) may be associated with a higher risk of ESRD, especially in female patients. Additionally, a significantly higher risk of ESRD in patients with a history of more serious infections requiring hospitalizations, including pneumonia, peritonitis, UTI, cellulitis, and bacteremia, was noted. These findings demonstrated a higher risk of ESRD in nephrotic patients with either frequent ARI or a history of bacterial infections requiring hospitalization. Our results confirmed the higher prevalence of ESRD in patients with NS with infections compared with those without infections, with the highest risk occurring in patients with more serious infections requiring hospitalization.
Infection is prevalent in people presenting with NS, and majority of infections have been reported to be associated with active disease in nephrotic patients (13). The increased frequency of infections may be due to the loss of immunoglobulins, impaired cellular immunity, and relative malnutrition (14). Studies have reported the relevance of UTI in patients with NS, and it was similar as ARI, both of which are the most frequent infectious triggers of relapse (15,16). In this study, admissions due to various infections, including UTI, was also demonstrated to be significantly associated with a higher risk of ESRD. Control of infection may help remission in some patients, and avoiding potential sources of infection may also reduce adverse renal outcome (17).
The findings of this study revealed a significantly higher prevalence of AKI in patients with NS with progression to ESRD. Occurrence of AKI during an infective episode was known, which is an established independent risk factor for kidney disease progression (18,19). Although AKI is a reversible condition, it may also entail the development of chronic kidney disease in some patients (20). In accordance with these findings, our data demonstrated that AKI has a significant risk of adverse renal outcome. Furthermore, cardiovascular disorders, including hypertension and CHF, were associated with an increased risk of ESRD, as indicated by our results.
A high cumulative dose of prednisone has been reported as a considerable risk factors for severe infections, and small increases in prednisolone dose can prevent relapse in upper respiratory tract infections in patients with NS (21,22). Based on our results, cautious prescription of prednisolone and efforts for reducing episodes of infection may help patients with NS to avoid progression to ESRD. Additionally, these data imply mandatory prophylactic interventions to prevent any infection in children and adults with NS. Some preventive methods have been proposed to reduce the risk of infection in NS in clinical practice (23,24).
In this study, we explored the occurrence of infections in nephrotic cases by using a nationally representative sample. Thereby, we demonstrated that (I) infections are associated with a significantly increased risk of ESRD in nephrotic patients, (II) hospitalization for bacterial infections is a strong risk factor for such morbidity, and (III) cardiovascular disorders and AKI are independent risk factors for kidney disease progression in the majority of patients with NS. Apart from various parameters examined clinically, our study suggests that it can be possible to predict patients with NS who are likely to develop ESRD later. Alternatively, our findings are also in accordance with other studies that suggest decreased kidney function to be associated with a significant high risk of serious infection (25,26). More importantly, NS was characterized by immunological abnormalities with T-cell imbalance and hypogammaglobulinemia, which were involved in the interaction between the virulence of the infecting organism and host defense mechanisms (27,28). The most commonly isolated bacterial species in the UTI were Escherichia coli (28%), and Klebsiella spp. (22.4%) (29). Moreover, the leading cause of bacterial peritonitis and sepsis in patients with NS was Streptococcus pneumoniae, a widespread pathogen that can cause pneumonia and other infectious complications (30). Additional studies to investigate the underlying immunological mechanism and limit infectious complications in patients with NS are required.
This is the first study to report that occurrence of infections adversely affect renal prognosis in nephrotic patients. The findings of our study revealed that the incidence of infections was independently associated with an increased rate of ESRD in patients with NS. Patients with NS have a high risk of infections, which are subsequently associated with adverse renal outcomes, especially in female and elderly patients.
Acknowledgments
Funding: This work was supported by China Medical University (CMU107-S-06). This study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance (Department of Health, Taiwan), which is managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Research Ethics Committee of Ditmanson Medical Foundation Chia-yi Christian Hospital (CYCH-IRB-106084).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shearer GC, Stevenson FT, Atkinson DN, et al. Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int 2001;59:179-89. [Crossref] [PubMed]
- Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ 2008;336:1185-9. [Crossref] [PubMed]
- Woo KT, Chan CM, Mooi CY, et al. The changing pattern of primary glomerulonephritis in Singapore and other countries over the past 3 decades. Clin Nephrol 2010;74:372-83. [Crossref] [PubMed]
- Chiu HF, Chen HC, Lu KC, et al. Distribution of glomerular diseases in Taiwan: preliminary report of National Renal Biopsy Registry-publication on behalf of Taiwan Society of Nephrology. BMC Nephrol. 2018;19:6. [Crossref] [PubMed]
- Charlesworth JA, Gracey DM, Pussell BA. Adult nephrotic syndrome: non-specific strategies for treatment. Nephrology (Carlton) 2008;13:45-50. [Crossref] [PubMed]
- McCaffrey J, Lennon R, Webb NJ. The non-immunosuppressive management of childhood nephrotic syndrome. Pediatr Nephrol 2016;31:1383-402. [Crossref] [PubMed]
- Adeleke SI, Asani MO. Urinary tract infection in children with nephrotic syndrome in Kano, Nigeria. Ann Afr Med 2009;8:38-41. [Crossref] [PubMed]
- Han JW, Lee KY, Hwang JY, et al. Antibody status in children with steroid-sensitive nephrotic syndrome. Yonsei Med J 2010;51:239-43. [Crossref] [PubMed]
- van den Berg JG, Weening JJ. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci (Lond) 2004;107:125-36. [Crossref] [PubMed]
- Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan. Results from a natural experiment. JAMA 1997;278:89-93. [Crossref] [PubMed]
- Zhang H, Wang Z, Dong L, et al. New insight into the pathogenesis of minimal change nephrotic syndrome: Role of the persistence of respiratory tract virus in immune disorders. Autoimmun Rev 2016;15:632-7. [Crossref] [PubMed]
- Zhai S, Hu L, Zhong L, et al. Respiratory syncytial virus aggravates renal injury through cytokines and direct renal injury. Front Cell Infect Microbiol 2016;6:112. [Crossref] [PubMed]
- Moorani KN, Khan KM, Ramzan A. Infections in children with nephrotic syndrome. J Coll Physicians Surg Pak 2003;13:337-9. [PubMed]
- Kemper MJ, Altrogge H, Ganschow R, et al. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol 2002;17:413-7. [Crossref] [PubMed]
- Uwaezuoke SN. Steroid-sensitive nephrotic syndrome in children: triggers of relapse and evolving hypotheses on pathogenesis. Ital J Pediatr 2015;41:19. [Crossref] [PubMed]
- Narain U, Gupta A. Urinary tract infection in children with nephrotic syndrome. Pediatr Infect Dis J 2018;37:144-6. [Crossref] [PubMed]
- Alwadhi RK, Mathew JL, Rath B. Clinical profile of children with nephrotic syndrome not on glucorticoid therapy, but presenting with infection. J Paediatr Child Health 2004;40:28-32. [Crossref] [PubMed]
- Maas RJ, Deegens JK, Beukhof JR, et al. The clinical course of minimal change nephrotic syndrome with onset in adulthood or late adolescence: a case series. Am J Kidney Dis 2017;69:637-46. [Crossref] [PubMed]
- Menon S. Acute kidney injury in nephrotic syndrome. Front Pediatr 2019;6:428. [Crossref] [PubMed]
- Yaseen A, Tresa V, Lanewala AA, et al. Acute kidney injury in idiopathic nephrotic syndrome of childhood is a major risk factor for the development of chronic kidney disease. Ren Fail 2017;39:323-7. [Crossref] [PubMed]
- Li J, Zhang Q, Su B. Clinical characteristics and risk factors of severe infections in hospitalized adult patients with primary nephrotic syndrome. J Int Med Res 2017;45:2139-45. [Crossref] [PubMed]
- Kershaw D. Small increases in prednisolone can prevent relapse during upper respiratory infections in patients with nephrotic syndrome. J Pediatr 2008;153:146-7. [Crossref] [PubMed]
- Shroff A, Frank R, Vergara M, et al. Prevention of serious bacterial infections in new-onset nephrotic syndrome: a survey of current practices. Clin Pediatr (Phila) 2002;41:47-9. [Crossref] [PubMed]
- Zou C, Su G, Wu Y, et al. Astragalus in the prevention of upper respiratory tract infection in children with nephrotic syndrome: evidence-based clinical practice. Evid Based Complement Alternat Med 2013;2013:352130. [Crossref] [PubMed]
- Dalrymple LS, Katz R, Kestenbaum B, et al. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis 2012;59:356-63. [Crossref] [PubMed]
- Ishigami J, Matsushita K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin Exp Nephrol 2019;23:437-47. [Crossref] [PubMed]
- Stachowski J, Barth C, Michałkiewicz J, et al. Th1/Th2 balance and CD45-positive T cell subsets in primary nephrotic syndrome. Pediatr Nephrol 2000;14:779-85. [Crossref] [PubMed]
- Yang X, Tang X, Li T, et al. Circulating follicular T helper cells are possibly associated with low levels of serum immunoglobulin G due to impaired immunoglobulin class-switch recombination of B cells in children with primary nephrotic syndrome. Mol Immunol 2019;114:162-70. [Crossref] [PubMed]
- Sorkhi H, Riahi SM, Ebrahimpour S, et al. Urinary tract infection in children with nephrotic syndrome: a systematic review and meta-analysis. Microb Pathog 2019;137:103718. [Crossref] [PubMed]
- Pittet LF, Posfay-Barbe KM, Chehade H, et al. Optimizing seroprotection against pneumococcus in children with nephrotic syndrome using the 13-valent pneumococcal conjugate vaccine. Vaccine 2016;34:4948-54. [Crossref] [PubMed]


