
Efficacy of Ca2+- or PO43−-conjugated mesoporous silica nanoparticles on dentinal tubule occlusion: an in-vitro assessment
Introduction
Prosthesis is a widely accepted dental procedure to repair a larger area of tooth defect. However, the preparation of vital teeth often leads to dentin exposure, even very close to the pulp cavity. In addition, toxic materials released from bacteria and external temperature stimulus from temporary crown prostheses could result in dentin hypersensitivity as well as pulpitis under certain conditions (1-3). Cutting, caries, attrition, abrasion and erosion result in dentinal tubule exposure followed by dentin hypersensitivity, which is commonly seen in dental practice (4,5).
One of the main treatments for dentin hypersensitivity is dentin tubule occlusion (6). For this treatment, chemical therapy, dental material filling and laser therapy are the most commonly used methods in dental clinics (7,8), where chemical therapy is the most frequently used. Unfortunately, the effectiveness of these therapies is short term since physical friction of daily tooth brushing and chewing as well as acid corrosion of food and drinks could lead to dentinal tubule dilation and/or gradual loss of tubule-occluding materials (9). Thus, it is crucial to develop a novel material with excellent resistance to daily acid corrosion and friction for effective occlusion of dentinal tubules.
Mesoporous silica nanoparticles (MSNs) demonstrate many superior properties: stable mesh structure, high surface energy, large surface area, high penetration rates, high solubility and high reaction activity (10-14). Thus, MSNs can effectively diffuse into narrow dentinal tubules of 2–3 µm in diameter. In addition, MSNs are able to attach tightly onto the tooth surface due to their high binding affinity and basic OH− groups (15). All these excellent properties make MSNs one of the best carriers (16). Considering the fact that the main component of human teeth is hydroxyapatite (HAP) (17), we proposed conjugating Ca2+ or PO43− with MSNs to form more biocompatible Ca2+@MSN and PO43−@MSN complexes. This MSN complex (Ca2+/PO43−@MSNs) is expected to deeply penetrate into the dentinal tubules, and a gradual release of Ca2+ and PO43− could form a stable phosphate calcium precipitate to seal the dentinal tubules (18). In this study, a Ca2+/PO43−@MSN complex was prepared. The features of the MSNs were characterized by transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The surface morphology and chemical compositions of Ca2+@MSNs/PO43−@MSNs and Ca2+/PO43−@MSNs were examined by SEM and X-ray fluorescence (XRF). The element distribution of Ca2+/PO43−@MSNs was detected using energy dispersive spectrometer (EDS). The sustained release ability was detected by inductively coupled plasma atomic emission spectrometry (ICP-AES). The optimal water–powder ratio for sealing dentinal tubules was determined. Compared with that of NovaMin (NovaMin Technology, Wuhan, China), one of the most commonly used materials in current dental practice, the capability of Ca2+/PO43−@MSNs to resist acid corrosion and physical friction was evaluated.
Methods
Preparation of Ca2+- or PO43−-conjugated MSNs
MSNs were prepared following a previously reported protocol (18). In brief, tetraethyl orthosilicate (TEOS) was used as a raw material, and hexadecyl trimethyl ammonium bromide (CTAB) was used as a template to prepare SiO2 microspheres under weakly alkaline conditions. We reduced the diameter of the particles by increasing the amount of water (from 420 to 480 mL) and prolonging the reaction time (from 2 to 3 h). The shape and structure of the MSNs were examined using TEM (HT-7700, Hitachi, Japan) at 100 kV and SEM (S-4800, Hitachi, Japan) at 10 kV. To conjugate MSNs with either Ca2+ or PO43−, 1.5 g MSNs were mixed with 2.8 g anhydrous calcium chloride and 0.3 g oxalic acid or with 2.7 g phosphoric acid (30%). Deionized water was then added, and the mixture was stirred gently. Next, the mixture was then dried in a drying oven at 105 °C for 4 h, transferred into a muffler furnace and heated at 200 °C for 4 h. The mixtures were finally cooled to room temperature and ground into fine powders. The surface appearance of Ca2+@MSNs, PO43−@MSNs and Ca2+/PO43−@MSNs was examined with SEM at 10 kV. The element distribution of Ca2+/PO43−@MSNs was detected using EDS. The chemical compositions of Ca2+@MSNs/PO43−@MSNs were analyzed using XRF (XRF-1800, Shimadzu, Japan).
Detection of the sustained release of Ca ions and P ions in vitro
Ca2+@MSNs (100 mg) or PO43−@MSNs (100 mg) were dissolved in 10 mL Tris-buffered saline (TBS, 50 mM Tris-HCl, 150 mM NaCl, and 0.02% NaN3, pH 7.4) solution in a centrifuge tube at 37 °C. The tubes were centrifuged at 4,000 rpm for 10 min, and the supernatant was collected for analysis. The separate sampling points were set at 0.5, 1, 2, 4, 8, 12, 24, 48 and 72 h. The Ca and P ions in the sampled supernatants were determined using ICP-AES (IRIS Intrepid XSP, Thermo Fisher Scientific Inc, USA).
Preparation of dentin discs
Caries-free premolars, clinically extracted for orthodontic treatment, were collected. The study was approved by our institutional review board (IRB K2016011), and written informed consent was obtained from all subjects. Each tooth was sectioned 1 mm above the cementoenamel junction perpendicular to the long axis of the tooth using a low-speed, water-cooled diamond saw. The dentin discs (1.0±0.1 mm in thickness) were polished stepwise with sandpapers at mesh sizes of 400, 600, 800, 1,000 and 1,200. These polished discs were immersed in 6% citric acid solution for 2 min and cleaned twice by ultrasonication in distilled water. These cleaned discs were stored in artificial saliva at 4 °C for use.
Optimization of different water-powder ratios
Five groups of different water–powder ratios were prepared (Table 1). All dentin discs were examined under a light microscope to ensure the consistency of the samples. Five dentin discs were selected, and each disc was cut into five equal parts that were randomly assigned to five groups and coated with Ca2+/PO43−@MSNs of different water-powder ratios. After mixing Ca2+@MSNs/PO43−@MSNs with deionized water, the material was immediately brushed on the surface of the selected discs, and this brushing process was repeated 20 s later. These discs were immersed in a solution of 3% glutaraldehyde overnight, dehydrated with gradient concentrations of ethyl alcohol, and sprayed with gold in vacuum after drying for SEM observation. The sealing rate of dentinal tubules in all discs was calculated using Image-Pro Plus 6.0.

Full table
Evaluation of the sealing effect under simulated conditions of acid corrosion and physical friction
Six dentin discs were randomly divided into two groups: the Ca2+/PO43−@MSN group (discs coated with the optimal concentration of Ca2+/PO43−@MSNs: Ca2+@MSNs: PO43−@MSNs: H2O =0.015 g: 0.015 g: 150 µL) and the NovaMin group (discs coated with NovaMin). For NovaMin (SiO2, Na2O, CaO, and P2O5), the application procedure followed the manufacturer’s instructions. Each disc was cut into three equal parts for the untreated disc test, acid corrosion test, and friction test. For the untreated test, the discs were stored in artificial saliva. For the acid corrosion test, the dentin discs were soaked in fresh Coca-Cola (pH 2.5) for 10 min every day to mimic the oral acidic environment. For the friction test, all the dentin discs were brushed by the same operator using a Colgate soft bristle toothbrush (a kind of toothbrush commonly used worldwide) with the same strength as was used in daily brushing. The dentin discs were brushed twice a day for 3 min each to mimic the oral friction environment. The discs for acid corrosion test and friction test were stored in artificial saliva daily. All discs were examined by SEM after one week of treatment. The sealing rates of dentinal tubules from both the Ca2+/PO43−@MSN and NovaMin groups under each treatment condition were calculated using Image-Pro Plus 6.0.
Statistical analysis
SPSS 21.0 was used for statistical analysis. The data are expressed as the mean ± standard deviation. One-factor ANOVA was used for the statistical analysis of the data when testing the different water-powder ratios, and multiple comparisons were conducted by Tukey’s test. Student’s t-test was used to compare two independent groups of data in the evaluation of the sealing effect under the simulated conditions of acid corrosion and physical friction. The significance level was set at α=0.05.
Results
Characterizations of MSNs and Ca2+/PO43−@MSNs
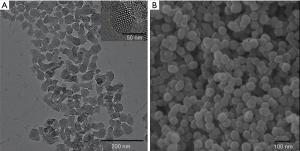
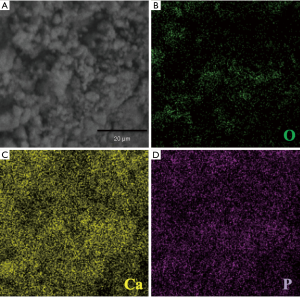
The TEM and SEM results showed that MSNs exhibited regular agglomeration and excellent dispersion ability, with even-sized particles and nearly spherical structure with a diameter of approximately 50 nm. The distribution of the inner core was also even and similar in size (Figure 1). SEM images (Figure 2) revealed that the surface of the Ca2+@MSNs/PO43−@MSNs was relatively rough [(A) Ca2+@MSNs; (B) PO43−@MSNs; (C) Ca2+/PO43−@MSNs]. Figure 2C showed a significant decrease in particle diameter due to the ion release. The EDS map (Figure 3) of Ca2+/PO43−@MSNs demonstrated that Ca, P, O elements were evenly distributed. The XRF results (Table 2) of the Ca2+@MSNs and PO43−@MSNs provided evidence that SiO2 and CaO as well as SiO2 and P2O5 were mainly present, respectively. The data showed that the loading rate was 38.976–41.227%.


Full table
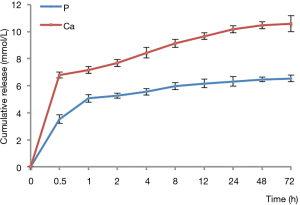
Release of Ca and P ions in vitro
Figure 4 shows that the release of Ca and P ions was rapid in the first 1 h and then gradually slowed until the end of the experiments. The Ca/P ratio of the new material at 72 h was 1.61, which is close to the Ca/P ratio of HAP.
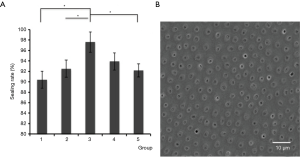
Effect of different water–powder ratios of Ca2+/PO43−@MSNs on dentinal tubule occlusion
Ca2+/PO43−@MSNs demonstrated an excellent effect on dentinal tubule occlusion at all five different concentrations but with variable efficacies depending on the concentration (Figure 5). Among the five groups, group 3 with Ca2+@MSNs: PO43−@MSNs: H2O =0.015 g: 0.015 g: 150 µL showed the best sealing effect, 97.6%.
Sealing effect under simulated conditions of acid corrosion and physical friction
As shown in Figure 6, although both Ca2+/PO43−@MSNs and NovaMin had different degrees of resistance to acid corrosion and friction, Ca2+/PO43−@MSNs exhibited better results than NovaMin. After acid etching treatment, we observed demineralization on the surface of the dentin discs. The dentinal tubules in the Ca2+/PO43−@MSN group remained well sealed, while most of the dentinal tubules in the NovaMin group were reopened. After friction treatment, the sealing rates of dentin discs in the Ca2+/PO43−@MSN and NovaMin groups were 88.6% and 71.7%, respectively.
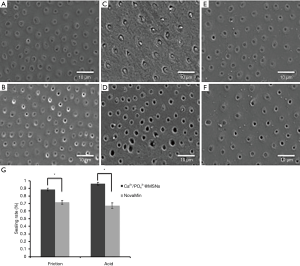
Discussion
The occlusion of dentinal tubules has become the preferred treatment for reducing dentin hypersensitivity, which is supported by the widely accepted hydrodynamics theory (19). Nanomaterials are promising for sealing dentinal tubules. One of the most commonly studied materials for such purposes is MSNs (18,20-22).
At present, most of the clinically applied tubule-occluding materials are immediate-release materials, generally resulting in the sealing materials accumulating only at the orifice of dentinal tubules. In comparison with other tubule-occluding materials, the Ca2+/PO43−@MSNs used in this experiment exhibit potential advantages of deeper and firmer sealing effects, which might be attributed to the following three reasons. First, the diameter of Ca2+@MSNs/PO43−@MSNs was much smaller than that of dentinal tubules, so the material could easily penetrate into the dentinal tubules. In our previous study, Ca2+ and PO43− were loaded into the pores of MSNs with diameters of 80 nm, with loading rates of 2.662–3.089% (18). In the current experiment, the diameter of MSNs was reduced to 50 nm for a deeper sealing effect, and Ca2+/PO43− was loaded on the surface of the particles and inside the pores, with an improved loading rate of 38.976–41.227%.
Second, owing to the sustained release capacity of the material, Ca2+ and PO43− were continuously released during the process of particle penetration for at least 72 h in this experiment, forming a stable calcium phosphate precipitate to continue consolidating the sealing effect. The initial pH of the composite is 4.7. Under this acidic condition, the dentin surface is likely to be slightly dissolved, and calcium ions from the dissolved dentin surface react with phosphate ions in the Ca2+/PO43−@MSNs to form precipitates and facilitate the sealing effect. At the same time, the dentin surface can also be roughened to strengthen the bonding strength with Ca2+/PO43−@MSNs. Therefore, proper acidity is desirable to improve the sealing effect. Clinically, 30% phosphoric acid (pH <1) is used to etch both enamel and dentin, so the acidity of Ca2+/PO43−@MSNs is acceptable for teeth. Moreover, MSNs had a certain difference in the loading of calcium and phosphorus because MSNs were negatively charged in aqueous solution (23). Compared with negatively charged phosphate ions, MSNs could load more positively charged calcium ions. Therefore, when the ions were released, the content of calcium ions would be more than that of phosphate ions. Under the experimental conditions, Ca2+/PO43−@MSNs had a Ca/P ratio of 1.61 at 72 h, which was close to that of HAP (1.67). The formed calcium and phosphorus compounds depositing on the wall of dentinal tubules might promote tooth remineralization (24,25), which would improve the reliability and effectiveness of the curative effect on dentin hypersensitivity.
Third, the mechanical sealing of Ca2+/PO43−@MSNs combined with the chemical sealing of calcium and phosphorus facilitated the sealing effect. From the XRF results in this study, we know that the proportion of MSNs is approximately 52.265–55.70%, which is lower than before, but the material contains much more calcium and phosphorus instead. In the dentinal tubules, calcium and phosphate ions are gradually released and form a precipitate that plays an important role in sealing dentin tubules together with the MSNs. The MSNs function by mechanical stacking, and the gaps between the MSN particles will be filled with the calcium-phosphate precipitate. Our previous study showed that although MSNs can seal dentinal tubules, their aggregates can most likely collapse after mechanically sealing the dentinal tubules if the bottoms of the dentinal tubules are not fully filled and consolidated (18). In the case of Ca2+/PO43−@MSNs, calcium phosphate precipitation caused by the release of calcium and phosphate ions played a key role in consolidating the sealing effect together with MSNs. Therefore, in the long run, calcium and phosphorus ions could enhance the stability of the sealing material in the dentinal tubules.
Although nanoscale MSNs can be immediately dissolved in deionized water, the water–powder ratios may influence the sealing effect. Insufficient sealing of the exposed dentinal tubules was observed when lower concentrations were used. At higher concentrations, the viscosity of suspensions increases and liquidity deterioration may occur, which prevents the diffusion of Ca2+/PO43−@MSNs into the dentinal tubules. For the viscosity of the solution is directly proportional to the concentration of the dispersion according to Einstein’s formula.
where µm is the viscosity of the suspension, φ is the volume fraction of the dispersed system in suspension, and µ is the viscosity of the continuous phase liquid. We found that Ca2+/PO43−@MSNs at the concentration of Ca2+@MSNs: PO43−@MSNs: H2O =0.015 g: 0.015 g: 150 µL displayed the best sealing effect on dentinal tubules. These results illustrate that it is critical to optimize the concentration to achieve an excellent sealing effect, and the optimized concentration provides a solid foundation for future clinical practice.
NovaMin, a common clinical tubule-occluding material, has chemical components and mechanical properties that are similar to those of Ca2+/PO43−@MSNs in terms of sealing dentinal tubules (26,27). Therefore, NovaMin was chosen as the control in this experiment. In comparison, Ca2+/PO43−@MSNs with the optimal water–powder ratio demonstrated a better sealing of the dentinal tubules and a better resistance to daily oral acid corrosion and tooth brushing. The ability to resist chemical corrosion and mechanical friction is an important parameter to evaluate the utility of sealing dentinal tubules. The major component of dentin is HAP, which can be partially dissolved under acidic conditions. It is a rational assumption that dental materials with chemical components similar to those of dentin could be decomposed by oral conditions (28). There is a possibility of dentinal tubule dilation and loss of the tubule-occluding material. As shown by the SEM images, the dentin surface started to demineralize or wear out under the conditions of simulated daily oral acid corrosion or tooth brushing. If the tubule-occluding materials are not embedded deeply and firmly enough in the dentinal tubules, they will wear out and dissolve, and even the sealed dentinal tubules will reopen.
The in vivo evaluation of this Ca2+/PO43−@MSN material is under investigation, and the sealing effect will be reported soon.
Conclusions
In summary, we have developed a novel dental material, Ca2+/PO43−@MSNs. The optimal concentration of this material (Ca2+@MSNs: PO43−@MSNs: H2O =0.015 g: 0.015 g: 150 µL) demonstrates a good effect on dentinal tubule sealing and can resist simulated oral acid corrosion and daily oral friction. These results suggest the potential efficacy of this material in long-term dentinal tubule sealing to reduce dentin supersensitivity and indicate its feasibility for clinical utilization.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by an institutional ethics committee and followed the tenets of the Declaration of Helsinki (No. K2016011).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holland GR, Narhi MN, Addy M, et al. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol 1997;24:808-13. [Crossref] [PubMed]
- Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non- sensitive cervical dentine. J Clin Periodontol 1987;14:280-4. [Crossref] [PubMed]
- Dowell P, Addy M. Dentine hypersensitivity--a review. Aetiology, symptoms and theories of pain production. J Clin Periodontol 1983;10:341-50. [Crossref] [PubMed]
- West NX. Dentine Hypersensitivity: Preventive and Therapeutic Approaches to Treatment. Periodontol 2000 2008;48:31-41. [Crossref] [PubMed]
- Addy M. Etiology and clinical implications of dentine hypersensitivity. Dent Clin North Am 1990;34:503-14. [PubMed]
- Orchardson R, Gillam DG. The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain 2000;14:9-19. [PubMed]
- Kimura Y, Wilder-Smith P, Yonaga K, et al. Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 2000;27:715-21. [Crossref] [PubMed]
- Xiao S, Liang K, Liu H, et al. Effect of Water-Cooled Nd:YAG Laser on Dentinal Tubule Occlusion in vitro. Photomed Laser Surg 2017;35:98-104. [Crossref] [PubMed]
- Lee BS, Tsai HY, Tsai YL, et al. In vitro study of dentinal tubule occlusion with sol-gel DP-bioglass for treatment of dentin hypersensitivity. Dent Mater J 2007;26:52-61. [Crossref] [PubMed]
- Chen Y, Chen H, Shi J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater 2013;25:3144-76. [Crossref] [PubMed]
- Hao N, Li L, Tang F. Shape-mediated biological effects of mesoporous silica nanoparticles. J Biomed Nanotechnol 2014;10:2508-38. [Crossref] [PubMed]
- Li H, Wu X, Yang B, et al. Evaluation of biomimetically synthesized mesoporous silica nanoparticles as drug carriers: Structure, wettability, degradation, biocompatibility and brain distribution. Mater Sci Eng C Mater Biol Appl 2019;94:453-64. [Crossref] [PubMed]
- Fu C, Liu T, Li L, et al. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013;34:2565-75. [Crossref] [PubMed]
- Yamamoto E, Kuroda K. Preparation and controllability of mesoporous silica nanoparticles. Enzymes 2018;44:1-10. [Crossref] [PubMed]
- West NX, Hughes JA, Addy M. Dentine hypersensitivity: the effects of brushing toothpaste on etched and unetched dentine in vitro. J Oral Rehabil 2002;29:167-74. [Crossref] [PubMed]
- Zhang L, Sun H, Yu J, et al. Application of electrophoretic deposition to occlude dentinal tubules in vitro. J Dent 2018;71:43-8. [Crossref] [PubMed]
- Suge T, Ishikawa K, Kawasaki A, et al. Duration of dentinal tubule occlusion formed by calcium phosphate precipitation method: in vitro evaluation using synthetic saliva. J Dent Res 1995;74:1709-14. [Crossref] [PubMed]
- Tian L, Peng C, Shi Y, et al. Effect of mesoporous silica nanoparticles on dentinal tubule occlusion: An in vitro study using SEM and image analysis. Dent Mater J 2014;33:125-32. [Crossref] [PubMed]
- Braennstroem M, Astroem A. A study on the mechanism of pain elicited from the dentin. J Dent Res 1964;43:619-25. [Crossref] [PubMed]
- Yu J, Yang H, Li K, et al. Development of epigallocatechin-3-gallate-encapsulated nanohydroxyapatite/mesoporous silica for therapeutic management of dentin surface. ACS Appl Mater Interfaces 2017;9:25796-807. [Crossref] [PubMed]
- Chiang YC, Lin HP, Chang HH, et al. A mesoporous silica biomaterial for dental biomimetic crystallization. ACS Nano 2014;8:12502-13. [Crossref] [PubMed]
- Mitchell JC, Musanje L, Ferracane JL. Biomimetic dentin desensitizer based on nano-structured bioactive glass. Dent Mater 2011;27:386-93. [Crossref] [PubMed]
- Chou CC, Chen W, Hung Y, et al. Molecular elucidation of biological response to mesoporous silica nanoparticles in vitro and in vivo. ACS Appl Mater Interfaces 2017;9:22235-51. [Crossref] [PubMed]
- Utneja S, Talwar S, Nawal RR, et al. Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: An in vitro study. J Conserv Dent 2018;21:681-90. [Crossref] [PubMed]
- Daas I, Badr S, Osman E. Comparison between fluoride and nano-hydroxyapatite in remineralizing initial enamel lesion: An in vitro study. J Contemp Dent Pract 2018;19:306-12. [Crossref] [PubMed]
- Abbassy MA, Bakry AS, Alshehri NI, et al. 45S5 Bioglass paste is capable of protecting the enamel surrounding orthodontic brackets against erosive challenge. J Orthod Sci 2019;8:5. [Crossref] [PubMed]
- Lopes RM, Scaramucci T, Aranha ACC. Effect of desensitizing toothpastes on dentin erosive wear and tubule occlusion. An in situ study. Am J Dent 2018;31:177-83. [PubMed]
- Sehmi H, Olley RC. The effect of toothbrush abrasion force on dentine hypersensitivity in-vitro. J Dent 2015;43:1442-7. [Crossref] [PubMed]


