
Prognostic value of chronic hepatitis B virus infection in patients with breast cancer in a hepatitis B virus endemic area
Introduction
Chronic hepatitis B virus (HBV) infection has been recognized as an urgent public health problem due to high infection rates, with more than 240 million chronic HBV carriers worldwide (1). China is one of the countries with a high incidence of hepatitis B infection in the world, while South China is one of the regions with the highest rate of chronic HBV infection in China. The seroprevalence of hepatitis B surface antigen (HBsAg) is 10% to 12% in the general population in South China (2). Chronic HBV infection has been widely confirmed as a causative factor in chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC). However, in addition to HCC, it has also been reported that chronic HBV infection affects the progression of other tumors, including non-Hodgkin lymphoma (3,4), hepatic cholangiocarcinoma (5,6), leukemia (7), gastric cancer (8), nasopharyngeal cancer (9), and pancreatic cancer (10). For example, HBV-infected non-HCC cancer patients, such as nasopharyngeal (9) or pancreatic cancer (11), have significantly worse clinicopathological features and prognosis than uninfected patients.
However, the impact of chronic HBV infection on the clinicopathological features and prognosis of patients with breast cancer (BC) is unclear. Therefore, this study intends to investigate the impact of chronic HBV infection on the clinicopathological features and prognosis of patients with BC in an epidemic area of HBV.
Methods
Study population and data extraction
This study mainly retrospectively collected patients with BC who had undergone surgery at the Sun Yat-sen University Cancer Center (Guangzhou, China) from February 2008 to December 2010. A total of 1,904 patients with BC who were pathologically confirmed and had no distant metastasis were identified. Patients with missing basic information such as tumor staging and unknown follow-up were excluded. All patients signed the informed consent form, and the study was approved by the Ethics Committee of the Cancer Center of Sun Yat-sen University. The staging of the tumor was performed according to the 7th edition of the TNM staging system of the American Joint Committee on Cancer (AJCC).
Serological detection of HBV infection
Blood tests for HBV infection in this study were performed before surgery for BC. Briefly, HBV is detected by collecting blood samples, separating serum, and then measuring serum samples by enzyme-linked immunosorbent assay. To ensure the accuracy of the test, HBV testing is performed and quality controlled according to standard operating procedures.
Patient follow-up and statistical analysis
All patients were routinely followed up after surgery. Patients were followed up every 3 months for the first 2 years, and every 3 to 6 months for the 3rd to 5th years, and 1–2 times a year until the death after the 5th year. The duration of follow-up refers to the interval between the diagnosis of BC to death or the last follow-up. The median follow-up time for HBsAg-positive patients was 68.5 months, compared with 70 months for HBsAg-negative patients. The effect of chronic HBV infection on overall survival (OS) and hepatic metastasis-free survival (HMFS) was the main research indicator for this study. We calculated the interval from the first day of diagnosis to the death or the last follow-up as OS and calculated the interval from the first day of diagnosis to the clinical detection of liver metastases as HMFS.
SPSS software (version 21.0; SPSS Inc., Chicago, IL, USA) was adopted to perform most of the statistical analysis. Chi-square test was used to compare statistical differences in clinical and pathological variables between HBsAg-positive and HBsAg-negative patients. When we analyzed the effect of chronic HBV infection on patient survival, we used Kaplan-Meier survival analysis to plot the survival curve and the difference was compared using a log-rank test. Statistically significant variables after univariate analysis were further used in the multivariate analysis of the Cox proportional hazard model to test the independent significance of the variables. The standard for statistical significance is set to 0.05, and all P values are based on two-sided testing. Kaplan-Meier curves for OS and HMFS were plotted by SAS software (SAS Institute Inc. version 9.4, USA).
Results
A total of 212 (11.1%) of the 1,904 patients were seropositive HBsAg. HBsAg-positive and HBsAg-negative patients are similar in most clinicopathological features. Besides, there was no significant difference in the surgical approach between the two groups. However, the proportion of younger patients (age ≤35 years) in the HBsAg-positive group was higher (15.6% vs. 9.0%; P=0.003) compared with the HBsAg-negative group in patients with BC (Table 1). In addition, the premenopausal patients in the HBsAg-positive group also had a higher proportion than the HBsAg-negative group (70.8% vs. 61.2%; P=0.004) (Table 1). Finally, the percentage of patients with lymphovascular invasion in the HBsAg-positive group was significantly higher than that in the HBsAg-negative group (5.2% vs. 2.5%; P=0.042) (Table 1).
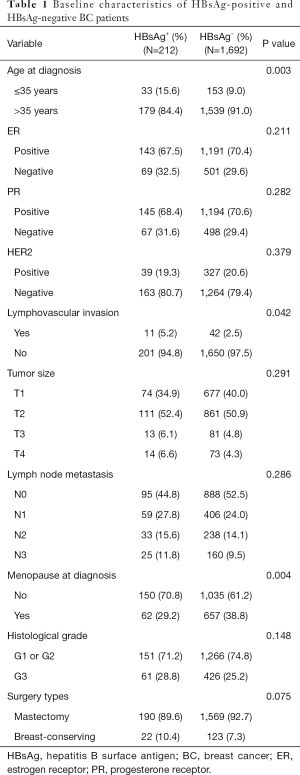
Full table
Effect of chronic HBV infection on the prognosis of patients with BC
The 5-year OS rate (84.9% vs. 90.4%, P=0.005) was significantly lower in HBsAg-positive BC patients than in HBsAg-negative patients (Figure 1A). Univariate analysis showed that the OS of HBsAg-positive BC patients was significantly worse than HBsAg-negative patients (Table 2). To adjust the influence of various confounding factors, Cox proportional hazards regression model is used for multivariate analysis. Multivariate analysis further determined that, chronic HBV infection is an independent risk factor for OS in patients with BC [hazard ratio (HR), 1.52; 95% confidence interval (CI), 1.02–2.26, P=0.038] (Table 2). Furthermore, the 5-year HMFS (92.5% vs. 97.1%, P=0.016) of patients with chronic HBV infection were significantly shorter compared with those without HBV infection (Figure 1B). In addition, later T and N staging were also independent risk factors for poor prognosis (Table 2).
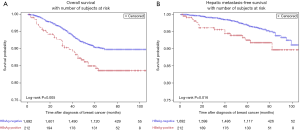
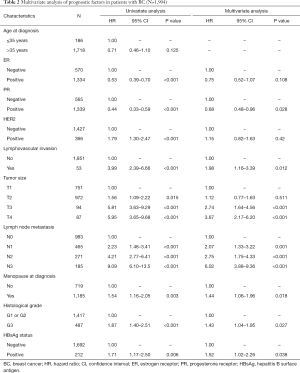
Full table
Effect of chronic HBV infection on survival outcome in BC patients with luminal or non-luminal BC
In patients with luminal BC, HBsAg-positive patients had worse OS compared with HBsAg-negative patients (85.7% vs. 91.7%; P=0.016) (Figure 2A). Multivariate analysis further confirmed that chronic HBV infection was an independent prognostic factor for OS in patients with luminal BC (HR, 1.62; 95% CI, 1.03–2.55; P=0.038). However, there is no significant association between chronic HBV infection and HMFS in luminal BC patients (Figure 2B). In non-luminal BC patients, chronic HBV infection also appeared to be associated with worse OS, but no statistical difference was observed (79.0% vs. 84.9%; P=0.139) (Figure 2C). Moreover, chronic HBV infection was significantly associated with poor 5-year HMFS (82.9% vs. 95.8%; P=0.002) (Figure 2D) in patients with non-luminal BC.
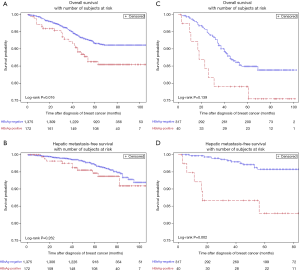
Effect of chronic HBV infection on survival outcome in BC patients with stage I or II/III
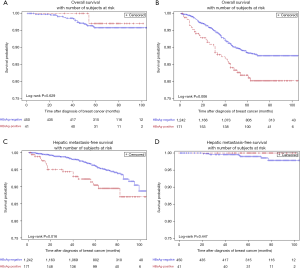
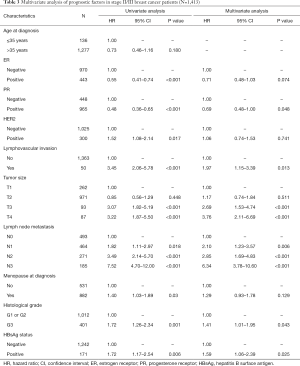
In stage I patients, no significant difference was observed in OS (95.8% vs. 97.1%; P=0.629) (Figure 3A) between HBsAg-positive patients and HBsAg-negative patients. In patients with stage II/III BC, HBsAg-positive patients had worse OS compared with HBsAg-negative patients (81.9% vs. 88.5%; P=0.006) (Figure 3B). Multivariate analysis further confirmed that chronic HBV infection was an independent prognostic factor for OS in patients with luminal BC (HR, 1.59; 95% CI, 1.06–2.39; P=0.025) (Table 3). Additionally, chronic HBV infection predicted a poor 5-year HMFS in stage II/III patients (90.5% vs. 96.3%; P=0.016) (Figure 3C), but not in patients with stage I BC (Figure 3D).
Full table
Discussion
To the best of our knowledge, the current study is the first large-scale study to determine the impact of chronic HBV infection in an endemic HBV region on the prognosis of patients with non-metastatic BC. The main finding of this study is that chronic HBV infection is an independent prognostic factor for stage II/III BC, but not stage I BC. In this cohort, the HBsAg positive rate of BC patients was 11.1%, and this infection rate was basically consistent with the general population in South China. We observed that young BC patients (less than or equal to 35 years old) accounted for a higher proportion of patients with chronic HBV infection than those older than 35 years. Furthermore, the proportion of premenopausal patients with chronic HBV infection is also higher in patients with BC than those without HBV infection. Another interesting finding is that patients with HBsAg-positive patients have a higher proportion of patients with lymphovascular invasion.
HBV mainly infects the liver and causes necrosis and inflammation of liver cells. In recent years, the impact of chronic HBV infection on cancer patients has received increasing attention. Previous studies have focused on the impact of HBV reactivation on cancer patients (12-15). In recent years, more and more studies have shown that chronic HBV infection can affect the prognosis of non-HCC cancer patients. Wang et al. found that compared with HBsAg-negative patients, diffuse large B-cell lymphoma patients with HBsAg-positive had a later clinical stage at the time of initial diagnosis (16). Liu et al. reported that chronic HBV infection was an independent risk factor for the survival of patients with locally advanced nasopharyngeal carcinoma (17). Wei et al. found that patients with HBV-infected pancreatic cancer had a worse prognosis and was significantly associated with an increased rate of simultaneous liver metastases (11).
Because the liver is most affected by HBV infection, does persistent HBV infection cause a microenvironment that is prone to liver metastasis? For the effect of chronic HBV infection on liver metastasis, the inconsistent conclusions have been reported in different tumors. It has been reported that chronic HBV infection increased the rate of simultaneous liver metastases in patients with pancreatic cancer but decreases the risk of liver metastasis in colorectal cancer (11,18). Although we found that 5-year HMFS (93.2% vs. 97.3%, P=0.016) was significantly worse in patients with chronic HBV infection than in those without HBV infection, multivariate analysis failed to confirm that chronic HBV infection independently affects HMFS. Therefore, whether HBV infection affects the occurrence of BC liver metastasis needs further research to verify.
The biological mechanisms by which chronic HBV infections affect BC prognosis observed in this study are still elusive. First of all, chronic HBV infection can damage liver cells and impair the deactivation of estrogen by hepatocytes (8,19). Persistent and long-term HBV infection in the liver impairs the normal function of the liver, which leads to elevated estrogen levels as it is primarily inactivated in the liver (20). This may explain to some extent that the observation of chronic HBV infection in this study mainly affects the prognosis of luminal BC, rather than other subtypes. Secondly, HBV may also directly affect breast cells through the action of oncoprotein HBV X protein (HBX) (21-23). For example, several studies have found that BC tissue highly expresses the oncoprotein HBXIP, a protein that interacts with HBX (24). Besides, chronic HBV infection may affect the host’s immune function, and it is reported that HBV is associated with immune dysfunction (25). The results of Li et al. revealed an HBV-induced immunosuppressive cascade in which HBV produces inhibitory monocytes that initiate regulatory NK cell differentiation leading to T cell suppression (26). Additionally, patients with chronic or regressive HBV infection are prone to complications of HBV reactivation during systemic therapy due to the immunosuppressive effects of administered chemotherapy. This may lead to liver damage, which may destroy the effect of anticancer treatment and affect the prognosis of patients (14,27). Most anti-cancer therapies, such as chemotherapy and radiation therapy, can cause immunosuppression, which can cause HBV reactivation and affect treatment (28,29). This may explain in part why the prognosis of patients with stage II/III complicated with chronic HBV infection is worse, as patients with stage II/III BC tend to receive chemotherapy, which may be harmful to the patient’s immune function. Lei et al. found that postoperative HBV reactivation is associated with increased postoperative complications and reduced survival in intrahepatic cholangiocarcinoma (30).
The results of this study provide the first evidence to be known as the poor prognosis of chronic HBV infection in patients with BC. Especially in areas with endemic chronic HBV infection, we should consider the impact of chronic HBV infection on the prognosis of patients with BC. We recommend that every BC patient in the HBV endemic area should have a serological test for HBV at the time of first admission and during the response assessment, whereas patients with serological HBsAg-positive should pay special attention to their possible adverse clinical outcomes. Due to the retrospective nature of this study, we were unable to examine the effect of HBV-DNA levels and antiviral therapy on the prognosis of patients with BC with chronic HBV infection. This is a major shortcoming of current research, and therefore, whether BC patients with higher HBV infection burden have poor survival remains unknown. With the increasing use of chemotherapy, targeted therapy, and endocrine therapy for systemic treatment of BC, the occurrence of HBV reactivation may increase during this period. However, there is a lack of data on clinical management of HBV screening and reactivation as well as BC patients with HBV infection. The difference in the risk of HBV reactivation in BC patients during different treatments and how to manage BC in the HBV endemic area deserves further study.
Conclusions
This study proved that chronic HBV infection was an independent risk factor for prognosis in patients with stage II/III BC. It is necessary to further confirm these results through large prospective studies, including the impact of HBV DNA load on BC prognosis. In addition, there is a need to investigate the underlying mechanisms of chronic HBV infection affecting the survival outcome of stage II/III BC patients.
Acknowledgments
We thank the staff of the Medical Records Management Section of the Sun Yat-sen University Cancer Center for supporting the research.
Funding: The study was funded by the National Natural Science Foundation of China (grant numbers: 81672598, 81772961).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Cancer Center of Sun Yat-sen University (No. GZR2016-076). All patients signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tang LSY, Covert E, Wilson E, et al. Chronic Hepatitis B Infection: A Review. JAMA 2018;319:1802-13. [Crossref] [PubMed]
- Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis 2010;14:1-21. vii. [Crossref] [PubMed]
- Lemaitre M, Brice P, Frigeni M, et al. Hepatitis B virus-associated B-cell non-Hodgkin lymphoma in non-endemic areas in Western Europe: Clinical characteristics and prognosis. J Infect 2020;80:219-24. [Crossref] [PubMed]
- Mahale P, Engels EA, Koshiol J. Hepatitis B virus infection and the risk of cancer in the elderly US population. Int J Cancer 2019;144:431-9. [Crossref] [PubMed]
- Clements O, Eliahoo J, Kim JU, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol 2020;72:95-103. [Crossref] [PubMed]
- Yang F, Ma L, Yang Y, et al. Contribution of Hepatitis B Virus Infection to the Aggressiveness of Primary Liver Cancer: A Clinical Epidemiological Study in Eastern China. Front Oncol 2019;9:370. [Crossref] [PubMed]
- Liang JH, Gao R, Dai JC, et al. The prognostic role of HBV infection in chronic lymphocytic leukemia. J Cancer Res Clin Oncol 2018;144:1309-15. [Crossref] [PubMed]
- Cui H, Jin Y, Chen F, et al. Clinicopathological evidence of hepatitis B virus infection in the development of gastric adenocarcinoma. J Med Virol 2020;92:71-7. [Crossref] [PubMed]
- Weng JJ, Wei JZ, Li M, et al. Effects of hepatitis B virus infection and antiviral therapy on the clinical prognosis of nasopharyngeal carcinoma. Cancer Med 2020;9:541-51. [Crossref] [PubMed]
- Desai R, Patel U, Sharma S, et al. Association Between Hepatitis B Infection and Pancreatic Cancer: A Population-Based Analysis in the United States. Pancreas 2018;47:849-55. [Crossref] [PubMed]
- Wei XL, Qiu MZ, Chen WW, et al. The status of HBV infection influences metastatic pattern and survival in Chinese patients with pancreatic cancer. J Transl Med 2013;11:249. [Crossref] [PubMed]
- Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer 2019;7:322. [Crossref] [PubMed]
- Yao ZH, Liao WY, Ho CC, et al. Incidence of hepatitis B reactivation during epidermal growth factor receptor tyrosine kinase inhibitor treatment in non-small-cell lung cancer patients. Eur J Cancer 2019;117:107-15. [Crossref] [PubMed]
- Yang HC, Tsou HH, Pei SN, et al. Quantification of HBV core antibodies may help predict HBV reactivation in patients with lymphoma and resolved HBV infection. J Hepatol 2018;69:286-92. [Crossref] [PubMed]
- Kusumoto S, Arcaini L, Hong X, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 2019;133:137-46. [Crossref] [PubMed]
- Wang F, Xu RH, Luo HY, et al. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B-cell lymphoma. BMC Cancer 2008;8:115. [Crossref] [PubMed]
- Liu X, Li X, Jiang N, et al. Prognostic value of chronic hepatitis B virus infection in patients with nasopharyngeal carcinoma: analysis of 1301 patients from an endemic area in China. Cancer 2014;120:68-76. [Crossref] [PubMed]
- Qiu HB, Zhang LY, Zeng ZL, et al. HBV infection decreases risk of liver metastasis in patients with colorectal cancer: A cohort study. World J Gastroenterol 2011;17:804-8. [Crossref] [PubMed]
- Jiang M, Klein M, Zanger UM, et al. Inflammatory regulation of steroid sulfatase: A novel mechanism to control estrogen homeostasis and inflammation in chronic liver disease. J Hepatol 2016;64:44-52. [Crossref] [PubMed]
- Raimondo G, Caccamo G, Filomia R, et al. Occult HBV infection. Semin Immunopathol 2013;35:39-52. [Crossref] [PubMed]
- Adhikari VP, Lu LJ, Kong LQ. Does hepatitis B virus infection cause breast cancer. Chin Clin Oncol 2016;5:81. [Crossref] [PubMed]
- Liu BW, Wang TJ, Li LL, et al. Oncoprotein HBXIP induces PKM2 via transcription factor E2F1 to promote cell proliferation in ER-positive breast cancer. Acta Pharmacol Sin 2019;40:530-8. [Crossref] [PubMed]
- Liu B, Wang T, Wang H, et al. Oncoprotein HBXIP enhances HOXB13 acetylation and co-activates HOXB13 to confer tamoxifen resistance in breast cancer. J Hematol Oncol 2018;11:26. [Crossref] [PubMed]
- Zhou XL, Zhu CY, Wu ZG, et al. The oncoprotein HBXIP competitively binds KEAP1 to activate NRF2 and enhance breast cancer cell growth and metastasis. Oncogene 2019;38:4028-46. [Crossref] [PubMed]
- Park JJ, Wong DK, Wahed AS, et al. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology 2016;150:684-95.e5. [Crossref] [PubMed]
- Li H, Zhai N, Wang Z, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut 2018;67:2035-44. [Crossref] [PubMed]
- Loomba R, Liang TJ, Hepatitis B. Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017;152:1297-309. [Crossref] [PubMed]
- Lv JW, Chen YP, Huang XD, et al. Hepatitis B virus screening and reactivation and management of patients with nasopharyngeal carcinoma: A large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer 2017;123:3540-9. [Crossref] [PubMed]
- Mücke MM, Backus LI, Mücke VT, et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018;3:172-80. [Crossref] [PubMed]
- Lei Z, Xia Y, Si A, et al. Antiviral therapy improves survival in patients with HBV infection and intrahepatic cholangiocarcinoma undergoing liver resection. J Hepatol 2018;68:655-62. [Crossref] [PubMed]


