Laparoscopic liver resection with simultaneous diaphragm resection
Introduction
Colorectal cancer is common in the Western countries (1). The most common site of distant metastases is the liver. About half of patients diagnosed with colorectal cancer will either present with synchronous liver metastases or subsequently develop liver metastases after definitive treatment of their primary tumor (2). For patients with isolated liver metastases, multimodal therapy involving surgery is the only potential curative treatment. Recent series have reported 5-year overall survival ranging 35–55% after liver resection for colorectal metastases combined with adjuvant and optionally neoadjuvant chemotherapy (3).
For colorectal liver metastases invading the diaphragm, liver resection with a simultaneous diaphragm resection is required to achieve negative surgical margins. However, resection of tumors with invasion of adjacent viscera is challenging. Simultaneous liver and diaphragm resection has been reported to be associated with higher morbidity rate and worse overall survival as compared with outcomes of liver resection alone (4-7).
Additionally, the need to resect the diaphragm is often realized by the surgeon intraoperatively, as tumor invasion of the diaphragm frequently is underestimated by the preoperative radiologic work-up (7,8). Differentiation between true invasion and dense adhesions liver adherence to the diaphragm is still difficult, even with the most advanced imaging techniques (8). This emphasizes the importance of having a clear routine to deal with diaphragm resection.
Most reports on this topic refer to open liver surgery, and laparoscopic simultaneous liver and diaphragm resection is thus uncommon and may be considered controversial (4-7,9-12).
During the recent 25 years, laparoscopy has tremendously changed the practice of gastrointestinal surgery (13-16). Besides, continuous improvement in imaging and systemic treatment regimens have altered both indications, techniques and outcomes in hepatic surgery (17).
The aim of this study was to assess the safety and value of simultaneous laparoscopic liver and diaphragm resection (SLLDR) in patients with colorectal liver metastases.
Methods
Patients, management, techniques
Oslo University Hospital is a high-volume institution for laparoscopic and open liver surgery. From August 1998 to January 2019 a total number of 1,233 LLR were performed in our institution, of whom 839 were performed for colorectal liver metastases. We here report outcomes from the cohort of patients that underwent SLLDR. The protocol has been approved by the institutional review board (protocol reference number 2015/13401).
Patients who underwent primary one-stage LLR for colorectal liver metastases from January 2008 to January 2019 were identified and included in this study. Patients that required simultaneous resection of other organs except the diaphragm or combined cryo- or radiofrequency ablations of the liver were excluded.
Patients who underwent SLLDR (group 1) were compared to patients who underwent LLR only (group 2). Standard preoperative investigations included abdominal and chest computed tomography and clinical biochemistry.
The surgical technique was described previously (18). Laparoscopic ultrasonography and presence of a range of advanced laparoscopic equipment were the prerequisites for LLR. An ultrasonic surgical aspirator, such as SonoSurg® (Olympus, Tokyo, Japan), or Selector®/CUSA® (Integra, Plainsboro, NJ, USA) and a bilobar coagulator LigaSure® (Medtronic, Minneapolis, MN, USA) were the main dissection instruments applied during the procedures.
If necessary, additional laparoscopic ports were introduced to facilitate resection of the diaphragm. A suction tip was introduced into the right pleural cavity via a laparoscopic port in all cases to evacuate pneumothorax. After diaphragm resection the defect was sutured by resorbable thread.
Postoperative analgesia consisted of a nonsteroidal anti-inflammatory drug and intravenous paracetamol. Opioids were given if additional analgesia was required. The patients were encouraged to mobilize early and resume feeding as soon as tolerated. Tumor size was measured following specimen fixation in formaldehyde during the histopathologic analyses of the resected specimens. Procedures with a resection margin ≥1 mm and no signs for residual tumor in the liver were considered as R0-procedures. Perioperative mortality was defined as death within 90 days or before hospital discharge.
Following diaphragm resection, patients had a plain thoracic X-ray direct postoperatively and on the first postoperative day to evaluate any residual pneumothorax.
Patients were routinely followed every 4 months up to 24 months and then every 6 months up to 60 months by outpatient visits with clinical examinations, carcinoembryonic antigen assay and imaging studies.
Statistics
Procedures were analyzed on intention to treat basis, i.e., cases converted to laparotomy were not excluded from the analyses. The data are presented as median (range), or number (percentage). To compare proportions between groups the Chi-square test or the Fisher exact test were used as appropriate. The Mann-Whitney test was used to compare continuous variables. The Life Tables and the Kaplan-Meier method were applied for survival analyses. Time defined survival values were presented in percentage ± standard error. Log-rank test was applied for comparison of survival between groups. Length of survival was described as mean [95% confidence interval (CI)].
Results
A total of 467 patients fulfilled the inclusion criteria, of whom 12 patients needed a simultaneous diaphragm resection (group 1) while 455 underwent laparoscopic liver surgery alone (group 2). Patient demographic data was similar in both groups (Table 1). The median follow-up was 31 [1–128], and 26 [0–110] months for the patients in group I, and II respectively.

Full table
All patients who had intraoperative suspicion of tumor invasion of the diaphragm underwent SLLDR. Only in 3 cases (25.0%) suspicion of tumor invasion was preoperatively based on CT and/or MRI. En bloc technique for SLLDR was applied in 10 out of 12 cases (83.3%). Histology confirmed the diaphragm invasion of the diaphragm in 9 cases (75.0%), including in all three cases of preoperative suspicion based on preoperative imaging. In both cases of SLLDR where en bloc techniques were not applied, histology confirmed that there was no tumor invasion of the diaphragm. Details of cases in the group 1 are summarized in Table 2. Overview of types of liver resections in the group 2 are presented in Table 3.
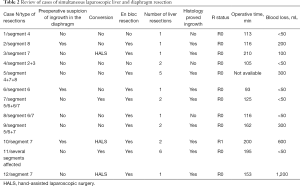
Full table

Full table
Two patients (16.7%) in group 1 were converted to open surgery, three additional patients were converted to hand-assisted laparoscopic surgery (HALS), whereas 11 patients (2.4%) were converted to laparotomy in group 2 (P=0.040) (Table 4).

Full table
Operative time and blood loss were similar in two groups. One patient in group 2 died within 30 days (severe bleeding from the right hepatic vein, expired in a multiorgan failure postoperatively), there was no mortality in group 1. The median tumor size (largest tumor) was 22 [8–40] mm in group 1 and 21 [3–110] mm in group 2 (P=0.977). The rate of R0 resections was 91.7% and 77.8% in groups 1 and 2 respectively (P=0.508). There was no statistical difference in the rate of postoperative complications and postoperative stay between the groups (Table 4).
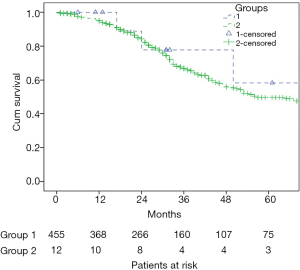
Six out of 12 patients in group 1 developed recurrences, including both hepatic and extrahepatic recurrences in five cases and recurrence in the lungs only in one case. Carcinomatosis developed in one case. There was no statistical difference between the groups in regard to overall survival rates (Table 5, Figure 1). Five-year actuarial overall survival was 58% and 49%, and mean overall survival was 89 (95% CI: 54–124) and 66 (95% CI: 61–71) months respectively.
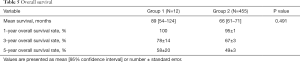
Full table
Discussion
Laparoscopic liver resection has been proven a good alternative to open liver resection for patients with colorectal liver metastases (14,15). A number of recent comparative studies, meta-analyses and two randomized trials have shown benefits of LLR over open liver resection in terms of perioperative outcomes, especially less morbidity and shorter hospital stay and equivalent oncologic outcomes (14-16,19).
In the present series we showed the technical feasibility, safety and efficacy of diaphragm resection during laparoscopic liver surgery. This is reflected in median operative time, blood loos, postoperative hospital stay and rate of postoperative morbidity compiling 125 minutes, 100 mL, 2.5 days and 8.3%—data that corresponds to LLR without diaphragm resection in our series. Diaphragm resection was performed without compromising oncologic outcomes, with a R0 resection rate of 92% and 5-year overall survival of 58%.
Irrespective to the operative approach, the impact of diaphragm invasion is sparsely studied. Only few small series and one multicenter database study have analyzed specifically the surgical results of simultaneous liver and diaphragm resection (4-7,9-12).
Some authors advocated blunt dissection to avoid unnecessary resection of the diaphragm, based on a low rate of reported true invasion to the diaphragm (20,21). In contrast, we applied en bloc techniques in 10 of the 12 SLLDR (83.3%). En bloc techniques was applied in all cases of dense adhesions of the liver to the diaphragm to avoid both tumoral rupture and bleeding due to parenchymal tear. R0 resection was obtained in 9 cases (90%) of en bloc SLLDR. Histology confirmed the tumor invasion to diaphragm in 9 cases (90%) of en bloc SLLDR. Thus, we suggest to perform diaphragm resection using an en bloc technique when a liver tumor is adhesive to the diaphragm, to avoid tumor rupture. In the two cases where en bloc resection was deemed unnecessary, histology confirmed the absence of tumor invasion.
It has been argued that in spite of improvements in imaging techniques, a reliable modality for preoperative diagnostics of tumor invasion to the diaphragm still does not exist (8). In our series imaging modalities showed only 33% of sensitivity to diagnose a tumor invasion to the diaphragm (preoperative radiologic suspicion in 3 out of 9 cases of histologically confirmed invasion), but 100% of specificity (all cases of preoperative radiologic suspicion were confirmed by histologic report). This underlines the importance of intraoperative diagnostics including intraoperative laparoscopic ultrasonography (22).
Technically, there are several challenging factors which have precluded surgeons from diaphragm resection during laparoscopic liver surgery. Tumors located in posterosuperior segments are more likely to grow into the diaphragm, and tumors in these segments are considered to be technically challenging (23,24). Patients with tumors in posterosuperior segments are still reserved for open liver resection in many institutions worldwide, although many leading hepatobiliary centers routinely perform LLR in these tumor locations (25,26).
However, SLLDR is technically more challenging to the surgeon than LLR only. This was reflected in a significantly higher rate of conversions to laparotomy in the group of SLLDR, 16.7% (2 cases) versus 2.4% in the group of LLR. Extensive adhesions were present in these two cases necessitating the conversions. There were also 3 (25%) additional cases of conversions to HALS. In all these three cases tumors were localized in the segment 7. We found HALS to be useful especially when performing diaphragm resection as it improves exposure and eases the closure of the defect in the diaphragm (27).
Development of pneumothorax might complicate good operative exposure. Different techniques can be applied to deal with this challenge. A transthoracic catheter may be utilized to evacuate the carbon dioxide from the pleural cavity, this allows retaining the diaphragm in a concave position (12). In our series, we have not considered this maneuver since the pneumothorax could be managed by placement of suction tip into the right pleural cavity. Some surgeons routinely apply a transthoracic trocar to improve surgical exposure when approaching tumors in the posterosuperior segments (28-32). In case of intraoperative finding that requires diaphragm resection this trocar port may be utilized for external suction of pneumothorax. We neither apply a transthoracic catheter or a transthoracic trocar, nor place thoracic drain and the end of the operation. In our experience, the residual pneumothorax dissolves rapidly and is usually not identifiable on the second postoperative day. It is possible that the routine use of a transthoracic trocar may prevent conversion to HALS. Interestingly, Lainas and colleagues reported a similar (14.3%) rate of conversions to open surgery in a series of seven patients undergoing SLLDR (12).
We consider the relatively high conversion rate in the SLLDR group as acceptable, as oncologic principles must prevail over the surgeon’s ambition to treat every patient with minimally invasive techniques. In view of the technical complexity of SLLDR, this procedure probably should be limited to expert centers.
Our study has several limitations. Most importantly, the number of patients in group 1 is low, so type 2 error may be present. The retrospective design is also a limitation, and there might be a selection bias where patients needing more complex diaphragm resections were operated with open liver resection. Further studies on this topic are required.
Conclusions
SLLDR can be performed safely with good short- and long-term outcomes in patients with colorectal liver metastases. We recommend en bloc resection when a liver tumor invades the diaphragm, and we find hand-assisted laparoscopic surgery useful in difficult cases.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: AMK serves as an unpaid editorial board member of Annals of Translational Medicine from Dec 2018 to Nov 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol has been approved by the institutional review board (protocol reference number 2015/13401).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maida M, Macaluso FS, Ianiro G, et al. Screening of colorectal cancer: present and future. Expert Rev Anticancer Ther 2017;17:1131-46. [Crossref] [PubMed]
- Henrikson NB, Webber EM, Goddard KA, et al. Family history and the natural history of colorectal cancer: systematic review. Genet Med 2015;17:702-12. [Crossref] [PubMed]
- Jones RP, Poston GJ. Resection of Liver Metastases in Colorectal Cancer in the Era of Expanding Systemic Therapy. Annu Rev Med 2017;68:183-96. [Crossref] [PubMed]
- Lordan JT, Riga A, Worthington TR, et al. Early and long-term outcomes of patients undergoing liver resection and diaphragm excision for advanced colorectal liver metastases. Ann R Coll Surg Engl 2009;91:483-8. [Crossref] [PubMed]
- Li GZ, Turley RS, Lidsky ME, et al. Impact of simultaneous diaphragm resection during hepatectomy for treatment of metastatic colorectal cancer. J Gastrointest Surg 2012;16:1508-15. [Crossref] [PubMed]
- Li GZ, Sloane JL, Lidsky ME, et al. Simultaneous diaphragm and liver resection: a propensity-matched analysis of postoperative morbidity. J Am Coll Surg 2013;216:402-11. [Crossref] [PubMed]
- Arkadopoulos N, Kyriazi MA, Perelas A, et al. Should diaphragmatic involvement preclude resection of large hepatic tumors? World J Surg 2013;37:2197-201. [Crossref] [PubMed]
- Karoui M, Tayar C, Laurent A, et al. En bloc stapled diaphragmatic resection for local invasion during hepatectomy: a simple technique without opening the pleural cavity. Am J Surg 2007;193:786-8. [Crossref] [PubMed]
- Shinke G, Noda T, Eguchi H, et al. Surgical outcome of extended liver resections for colorectal liver metastasis compared with standard liver resections. Mol Clin Oncol 2018;9:104-11. [PubMed]
- Hand F, Sanabria Mateos R, Durand M, et al. Multivisceral Resection for Locally Invasive Colorectal Liver Metastases: Outcomes of a Matched Cohort Analysis. Dig Surg 2018;35:514-9. [Crossref] [PubMed]
- Hadden WJ, de Reuver PR, Brown K, et al. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB (Oxford) 2016;18:209-20. [Crossref] [PubMed]
- Lainas P, Camerlo A, Conrad C, et al. Laparoscopic right hepatectomy combined with partial diaphragmatic resection for colorectal liver metastases: Is it feasible and reasonable? Surgery 2015;158:128-34. [Crossref] [PubMed]
- Edwin B, Nordin A, Kazaryan AM. Laparoscopic liver surgery: new frontiers. Scand J Surg 2011;100:54-65. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Yamazaki S, Takayama T. Current topics in liver surgery. Ann Gastroenterol Surg 2019;3:146-59. [Crossref] [PubMed]
- Kazaryan AM, Pavlik Marangos I, Rosseland AR, et al. Laparoscopic liver resection for malignant and benign lesions: ten-year Norwegian single-center experience. Arch Surg 2010;145:34-40. [Crossref] [PubMed]
- Robles-Campos R, Lopez-Lopez V, Brusadin R, et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 2019;33:3926-36. [Crossref] [PubMed]
- Ezaki T, Koyanagi N, Toyomasu T, et al. Appraisal of a manual blunt dissection for an intraoperative diagnosis of extrahepatic cancer invasion. Hepatogastroenterology 1998;45:1837-41. [PubMed]
- Yamashita Y, Morita K, Iguchi T, et al. Surgical impacts of an en bloc resection of the diaphragm for hepatocellular carcinoma with gross diaphragmatic involvement. Surg Today 2011;41:101-6. [Crossref] [PubMed]
- Ferrero A, Lo Tesoriere R, Russolillo N, et al. Ultrasound-guided laparoscopic liver resections. Surg Endosc 2015;29:1002-5. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Teo JY, Kam JH, Chan CY, et al. Laparoscopic liver resection for posterosuperior and anterolateral lesions-a comparison experience in an Asian centre. Hepatobiliary Surg Nutr 2015;4:379-90. [PubMed]
- Aghayan DL, Fretland ÅA, Kazaryan AM, et al. Laparoscopic versus open liver resection in the posterosuperior segments: a sub-group analysis from the OSLO-COMET randomized controlled trial. HPB (Oxford) 2019;21:1485-90. [Crossref] [PubMed]
- Morikawa T, Ishida M, Takadate T, et al. Laparoscopic partial liver resection improves the short-term outcomes compared to open surgery for liver tumors in the posterosuperior segments. Surg Today 2019;49:214-23. [Crossref] [PubMed]
- Fiorentini G, Swaid F, Cipriani F, et al. Propensity Score-Matched Analysis of Pure Laparoscopic Versus Hand-Assisted/Hybrid Major Hepatectomy at Two Western Centers. World J Surg 2019;43:2025-37. [Crossref] [PubMed]
- Ikeda T, Toshima T, Harimoto N, et al. Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc 2014;28:2484-92. [Crossref] [PubMed]
- Yamashita S, Loyer E, Kang HC, et al. Total Transthoracic Approach Facilitates LaparoscopicHepatic Resection in Patients with Significant Prior Abdominal Surgery. Ann Surg Oncol 2017;24:1376-7. [Crossref] [PubMed]
- Ichida H, Ishizawa T, Tanaka M, et al. Use of intercostal trocars for laparoscopic resection of subphrenic hepatic tumors. Surg Endosc 2017;31:1280-6. [Crossref] [PubMed]
- Fuks D, Gayet B. Laparoscopic surgery of postero-lateral segments: a comparison between transthoracic and abdominal approach. Updates Surg 2015;67:141-5. [Crossref] [PubMed]
- Chiow AK, Lewin J, Manoharan B, et al. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford) 2015;17:299-303. [Crossref] [PubMed]