
Risk of head and neck cancer after chronic pancreatitis
Introduction
Head and neck cancer (HNC) ranks as the seventh among the top common cancers globally, and approximately half a million cases are diagnosed annually (1). HNC consists of primary cancers originating from the paranasal sinuses, nasal cavity, salivary glands, oral cavity, pharynx, and larynx. Oral and laryngeal cancers are the commonest subtypes of HNCs, and Taiwan had an annual incidence of 22/100,000 for oral and pharyngeal cancers in 2011 (2). Tobacco and alcohol have been well-recognized to be closely associated with the development of HNCs (3). The incidence of HNC varies substantially by sex, age, ethnic, environment, socioeconomic situation, and comorbidities, such as male sex and low socioeconomic status are reportedly associated with increased risks of HNC (4). Studies have shown that not only the direct healthcare costs but also indirect costs due to substantial work loss are typically higher for patients with HNC (5). Therefore, recognizing high risk individuals for the development of HNC is important to ensure the successful prevention, detection, and management of this type of cancer.
Chronic pancreatitis (CP) can result in endocrine or exocrine insufficiency due to chronic progressive inflammation and irreversible pancreas damage (6). Heavy alcohol consumption, which is observed in approximately 70–80% of CP patients, is the most common etiology of CP (7). The positive correlation between smoking and CP is also highly consistent with a dose-dependent effect in epidemiologic studies (6). The clinical diagnostic standard for CP mainly depends on image findings of irreversible pancreas damage, such as calcifications, stones, and duct stricture or dilatation (8,9). The reported prevalence of CP ranges between 3 and 42 per 100,000 population, and investigations of chronic pancreatitis have mainly focused on the functional insufficiency of the pancreas, pancreatic cancer, chronic pain, and alcohol abstinence (9,10).
Chronic pain, the commonest symptom of CP, can profoundly decrease mental and physical quality of life (11,12). Therefore, most clinicians overlook the risk of HNC and deviate to the management of pancreatic insufficiency, abdominal pain, and the psychological effects of CP (6,10,12-15). The Taiwan government has provided the citizens with habits of tobacco or betel nut consumption a nationwide screening program for oral cancer since 2004 (16). Some CP patients with the aforementioned habits might have received oral cancer screening. However, there was no nationwide implementation of screening program for HNC in Taiwan and no literature has studied the risk of HNC for the CP patients. An evidence-based survey must be conducted to assess the risk of HNC in patients with CP. With the hypothesis that CP is associated with the development of HNC, we analyzed Taiwan’s National Health Insurance Research Database (NHIRD) to assess the aforementioned association. However, we cannot ascertain the pathogenesis in this population-based observational study.
Methods
Patient and public involvement
The Taiwan National Health Insurance (NHI) program has been launched since March 1, 1995 and has provided over 99.6% of the 23 million residents with healthcare in Taiwan. We used the claims data for hospitalization of the NHI program in Taiwan, the LHID2000 (Longitudinal Health Insurance Research Database 2000) of the NHIRD, to conduct this retrospective nationwide cohort study (17). The NHIRD was administered by the National Health Research Institutes for medical research (http://nhird.nhri.org.tw/) (18,19). We made the disease coding based on the 2001 International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) for disease coding. We identified the patients aged ≥20 years with newly identified CP (ICD-9-CM: 577.1) between 2000 and 2011 as the case cohort, and we appointed the date of CP identification as the index date. We randomly selected the individuals without known CP through 1:1 propensity score matching with the study cohort by sex, age (in 5-y intervals), occupation, urbanization level, index year, monthly income (New Taiwan dollar; NTDs), and comorbidities as the control cohort. The patients without known age or sex and those with identified malignancy (ICD-9-CM 140–208) were excluded from the CP and non-CP cohorts (as Figure 1). The disease event of this study was HNC (ICD-9-CM 140–149, 161). We follow each patient from the index date to the development of HNC, withdrawal from the NHI program due to death or emigration, or Dec. 31, 2011. Patients would be censored when the cause of death, either cause-specific and non-cause-specific, could not be identified. The death cause and the ascertainment of HNC was made based on the data retrieval from the Death Certificate Registry and the Catastrophic Illness Registry, respectively. The cause of death should be reported to the Death Certificate Registry, administered by the Ministry of the Interior, based on the doctor’s certification. Patients with HNC can apply for the exemptions of reimbursement copayment with the Catastrophic Illness Certificate, audited by the Taiwan Ministry of Health and Welfare (MOHW), based on the doctors’ certification and the medical records. The coding accuracy for the causes of death and cancer in Taiwan has been validated previously (20,21).
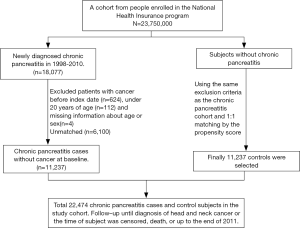
The comorbidities analyzed in this study included hyperlipidemia (ICD-9-CM 272), hypertension (ICD-9-CM 401–405), COPD (chronic obstructive pulmonary disease) (ICD-9-CM codes 491, 492, and 496), diabetes (ICD-9-CM 250), CAD (coronary artery disease) (ICD-9-CM codes 410–414), alcohol-related illness (ICD-9-CM 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3), stroke (ICD-9-CM 430–438), asthma (ICD-9-CM 493), and acute pancreatitis (ICD-9-CM 577.0).
Data sharing statement
We access the dataset after the approval of the Taiwan MOHW. The contact information of MOHW includes Address: No.488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.); Email: stcarolwu@mohw.gov.tw; and Phone: +886-2-8590-6848.
Ethics statement
The NHIRD encrypted all the personal information, such as claims information, including sex, date of birth, medical services received, and prescriptions, by anonymous identification numbers to waive the patient consent for accessing. This study has been approved by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-CR4).
Statistical analysis
We analyzed the distributions of age, sex, monthly income, urbanization level, occupation category, and comorbidities by the chi-squared test. We compared the mean ages (SD), frequency of medical visits (SD), and follow-up periods (SD) between the two cohorts by the Student’s t test. We used the Kaplan-Meier method to compare the HNC incidence and the log-rank test to examine the differences between the case and control cohorts. We stratified age, sex, occupation category, urbanization level, monthly income, and comorbidities to measure the incidence density rates of HNC and assessed the risk of HNC by univariable and multivariable Cox proportional hazards regression models. The Cox model estimated hazard ratios (HRs) and 95% confidence intervals (CIs) after adjustment for age, sex, frequency of medical visits, occupation, urbanization level, monthly income, and comorbidity of hyperlipidemia, hypertension, COPD, diabetes, CAD, alcohol-related illness, stroke, asthma, and acute pancreatitis. The software used for data analyses was SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
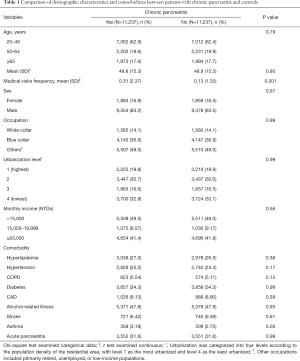
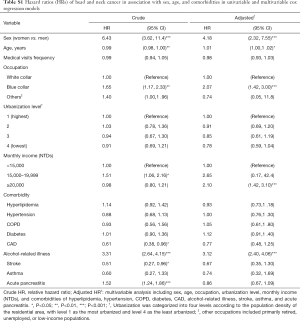
The comparison of the demographic characteristics and comorbidities between patients with and without CP is shown in Table 1. This study examined CP and non-CP cohorts, each consisting of 11,237 patients. The two cohorts were well matched for age, sex, occupation, urbanization level, monthly income, and comorbidity of hyperlipidemia, hypertension, COPD, diabetes, CAD, alcohol-related illness, stroke, asthma, and acute pancreatitis. The mean age was 48.8±15.3 years in the CP cohort and 48.9±15.3 years in the non-CP cohort, respectively. The mean frequency of medical visits (medical visits/years of follow-up) was 0.31 [standard deviations (SD) =2.37] in the CP cohort and 0.13 (SD =1.33) in the non-CP cohort, respectively. Most patients were 49 years or younger (62.9%), the majority were male (83.2%), and the prevalence of CP decreased with increasing age. In the study cohorts, the most common comorbidities were alcohol-related illness (47.8%), diabetes (34.3%), history of acute pancreatitis (31.6%), hyperlipidemia (27.0%), and hypertension (25.2%). There was no correlation between CP and monthly income or urbanization level.
Full table
The cumulative incidence of HNC was higher in the CP cohort than that in the non-CP cohort (log-rank test, P<0.001), with an average follow-up duration of 4.87±3.30 years for the CP cohort and 5.43±3.23 years for the non-CP cohorts (Figure 2).
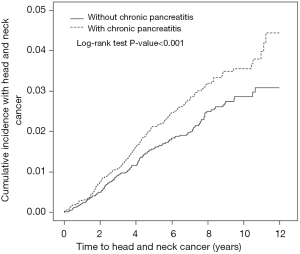
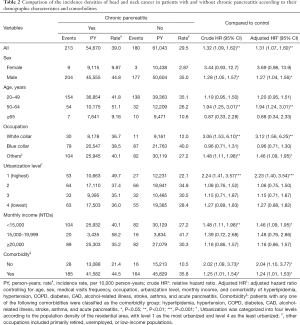
Table 2 compares the incidence densities of HNC between both cohorts based on the stratification of their demographic characteristics and comorbidities. Compared with the non-CP cohort, the CP cohort had an increased risk of HNC [adjusted HR (aHR) =1.31, 95% CI: 1.07–1.60] after controlling age, sex, frequency of medical visits, occupation, urbanization level, monthly income, and comorbidity of hyperlipidemia, hypertension, COPD, diabetes, CAD, alcohol-related illness, stroke, asthma, and acute pancreatitis. The experimental event rate (EER) of HNC for the CP cohort was 1.90% (213/11,237) and control event rate (CER) of HNC for the non-CP cohort was 1.60% (180/11,237), respectively. Therefore, the absolute risk increase (ARI) of HNC was 0.30% (EER-CER) and the number needed to harm (NNH) was approximately 333 (1/ARI) for the CP cohort. It should be noted that CP was consistently related to the development of HNCs (crude HR =1.33, 95% CI: 1.08–1.64; aHR =1.31, 95% CI: 1.06–1.61) after excluding the possible human papilloma virus (HPV)-related HNC with involvement of the oropharynx sites, such as soft palate, tonsil, tonsil arch, base of tongue, lateral wall and posterior wall (ICD-9-CM 141.0, 145.3, 145.4, 146). The EER of possible HPV-unrelated HNC for the CP cohort was 1.70% (191/11,215) and control event rate (CER) of HPV-unrelated HNC for the non-CP cohort was 1.44% (161/11,218), respectively. Therefore, the ARI of HPV-unrelated HNC was 0.26% and the NNH was approximately 385 for the CP cohort (data not shown).
Full table
CP had a greater contribution to the sex-specific relative risk of HNC for men (aHR =1.27, 95% CI: 1.04–1.56) but not for women (aHR =3.69, 95% CI: 0.98–13.9). Furthermore, CP had a greater contribution to the age-specific relative risk of HNC for middle-aged patients (20–49 years: aHR =1.20, 95% CI: 0.95–1.51; 50–64 years: aHR =1.94, 95% CI: 1.24–3.01; and ≥65 years: aHR =0.88, 95% CI: 0.34–2.33). Moreover, CP had a greater contribution to the relative risk of HNC for the patients with a white-collar job (aHR =3.12, 95% CI: 1.56–6.25), living in level 1 urbanization areas (aHR =2.23, 95% CI: 1.40–3.54), or with a monthly income < New Taiwan Dolars (NTDs) 15,000 (aHR =1.46, 95% CI: 1.09–1.95). However, among the occupation categories, it should be noted that blue-collar job remained as an independent risk factor (aHR =2.07, 95% CI: 1.42–3.00) for the development of HNC in multivariable Cox proportional hazards regression model; therefore, the contribution of CP to the development of HNC was insignificant for patients with blue-collar jobs (Table S1). The rate of HNC increased from 10.5/10,000 person-years for non-CP patients without comorbidities to 21.4/10,000 person-years for CP patients without comorbidities. Furthermore, the rate of HNC increased from 35.8/10,000 person-years for non-CP patients with comorbidities to 44.5/10,000 person-years for CP patients with comorbidities. Notably, the contribution of CP to the relative risk of HNC was greater for patients without comorbidities (aHR =2.04, 95% CI: 1.10–3.77) than those with comorbidities (aHR =1.24, 95% CI: 1.01–1.53).
Full table
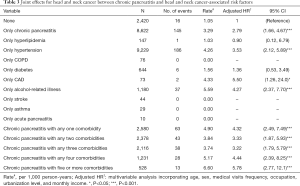
Table 3 shows the combined effects of CP and comorbidity on the development of HNC. The overall incidence density rates of HNC in the CP cohort without any comorbidity and non-CP cohort without any comorbidity were 3.29 and 1.05 per 10,000 person-years, respectively. Compared with the non-CP individuals without any other comorbidity, the risk of developing HNC in CP increased from 2.79 (95% CI: 1.66–4.67) when no comorbidities existed to 4.32 (95% CI: 2.49–7.49), 3.33 (95% CI: 1.87–5.93), 3.22 (95% CI: 1.79–5.79), 4.44 (95% CI: 2.39–8.25), and 5.78 (95% CI: 2.77–12.1) in the presence of any one, any two, any three, any four, and five or more comorbidities, respectively. In addition to only chronic pancreatitis (aHR =2.79, 95% CI: 1.66–4.67), among the comorbidities, multivariable analysis identified that only hypertension (aHR =3.53, 95% CI: 2.12–5.89), only CAD (aHR =5.50, 95% CI: 1.26–24.0), and only alcohol-related illness (aHR =4.27, 95% CI: 2.37–7.70) were the independent risk factors for HNC. Although there was no case of HNC in those patients with only acute pancreatitis, approximately 2.2% (156/7,103) of the patients with history of acute pancreatitis, either accompanied by or not accompanied by other comorbidities, finally developed HNC, including 76 cases in the 3,552 CP patients with history of acute pancreatitis and 80 cases in the 3,551 non-CP patients with history of acute pancreatitis. Although there was no case of HNC in those patients with only COPD, approximately 1.3% (15/1,197) of the patients with COPD, either accompanied by or not accompanied by other comorbidities, finally developed HNC, including 8 cases in the 623 CP patients with COPD and 7 cases in the 574 non-CP patients with COPD.
Full table
Discussion
The most common comorbidities observed in patients with CP included alcohol-related illness and history of acute pancreatitis (Table 1). Our findings support that alcohol consumption may be the main cause of CP, although NHIRD had no information on lifestyle or dietary habits. In addition to alcohol-related illness and history of acute pancreatitis, our findings support the association of CP with diabetes, hyperlipidemia, hypertension, and CAD (22,23). The possible pathogenesis for the aforementioned association may be related to alcohol consumption and the development of diabetes with consequent atherosclerosis.
CP remained closely associated with the development of HNC after controlling age, sex, frequency of medical visits, occupation, urbanization level, monthly income, and comorbidities of hyperlipidemia, hypertension, COPD, diabetes, CAD, alcohol-related illness, stroke, asthma, and acute pancreatitis in Cox proportional hazards regression models (Table 2). The cause of CP varies in different Asia-pacific countries (24,25). CP would develop in 10% of patients with initial diagnosis of acute pancreatitis and 36% of patients with recurrent attacks of acute pancreatitis, respectively (26). Furthermore, the course CP may be insidious without prior history of acute pancreatitis (27). We therefore control for acute pancreatitis in the matching and the analysis. However, CP was consistently related to the development of HNC (aHR =1.31, 95% CI: 1.07–1.59) even though we did not consider acute pancreatitis as a confounding factor in multivariable analysis (data not shown). We did not include the patients with pre-existing malignancy into our cohorts, and the selection bias remains debated about the exclusion or inclusion of the patients with pre-existing malignancy. Excluding patients with malignancy may underestimate the risk of HNC for CP since the CP cases will have had more cancers due to tobacco smoking, alcohol dinking, or hereditary liability. However, including patients with pre-existing malignancy will also raise the concern of underestimating the risk of HNC for CP since the CP cases may have died of more competing factors, including malignancy, before the development of HNC.
CP had a greater contribution to the development of HNC for male or middle-aged patients. Our findings were consistent with the literature in showing that CP had a greater contribution to the development of HNC for patients with low incomes (28,29). However, occupation and urbanization level could not consistently reflect the actual socioeconomic status of the individuals to demonstrate their close associations with HNC in CP patients. CP had a greater contribution to the development of HNC for patients without comorbidities than for patients with comorbidities even though the absolute risk of HNC was greater in the patients with comorbidities. Moreover, the risk of HNC for the CP cohort increased with the incremental follow-up duration irrespective shorter follow-up duration in the CP cohort (Figure 2). Notably, the risk or the rate of HNC for CP patients was particularly high in men, middle-aged patients, and patients with comorbidities. All these findings support CP was related to an increased risk of HNC in this observational study and indicate that enhancing HNC screening programs may be critical in male, middle-aged, and low-income patients with CP.
Among the comorbidities, our findings identified hypertension, CAD, and alcohol-related illness were associated with the development of HNC (Table 3). Cardiovascular disease (CVD) may share common molecular and genetic pathways with the pathogenesis of malignancy (30). First, inflammatory atherosclerotic plaque can enhance inflammatory signaling and gene expression for carcinogenesis. Second, atherosclerosis increases reactive oxygen species formation, inflammation, and proliferation of vascular smooth muscle cell. Third, fatty acid synthase is abundant in atherosclerotic plaque and is overexpressed in malignant tumors. Diabetes is thought to be associated with the development of HNC because insulin promotes carcinogenesis and hyperglycemia promotes tumor growth (31). However, our results were consistent with the literature reported from Taiwan in showing that diabetes had no relation to the development of HNC after adjustment for potential confounders (32). The main metabolites of alcohol, including ethanol and acetaldehyde, have been deemed as class I carcinogens and alcohol can act as a solvent for tobacco to enhance carcinogenesis (3). COPD was not associated with development of HNC in our study, and we acknowledge that COPD cannot completely represent smoking status, frequency, and duration to clarify the association between tobacco smoking and risk of HNC.
Although no literature to date have evaluated the relation of CP to HNC, we postulate the possible mechanisms for their association. First, CP and HNC share both tobacco and alcohol as the risk factors. Alcohol consumption is the main cause of CP, and there is a dose-dependent association between smoking and CP (6). Most HNCs, except oral cavity cancer and those in women or younger patients, are attributed to tobacco or alcohol use (33). Second, CP can provide inflammatory mediators, such as ROS and reactive nitrogen species, to enhance the process of inflammation-associated carcinogenesis (34). ROS can cause genomic mutation and serve as signaling molecules for tumor cell proliferation and angiogenesis.
Our population-based cohort study had several advantages. First, it used a longitudinal database to observe approximately 1,000,000 residents of Taiwan over 12 years in order to examine the relation of CP to the development of HNC. Our study with a large sample size and 12-year-long follow-up resulted in high statistical power and low selection bias. Second, more than 99.6% of Taiwan’s residents have been covered by the NHI program and our findings provided the generalization in Taiwan.
Our study had several limitations. First, the inherently unavailable information of the patients' smoking habits and alcohol drinking is the major limitation of this retrospective study. However, we have tried our best to adjust the potential tobacco-associated diseases, such as COPD, CAD, stroke, and asthma, in assessing the association between HNC and CP. We also controlled alcohol-associated illness, the commonest comorbidity for the CP patients, during our analysis. Among the CP patients, approximately 23.3% (2,615/11,237) had comorbidities and the risk of HNC was notably higher in the presence of comorbidity. Moreover, most of the comorbid diseases may be related to either smoking or drinking although the cases and controls are matched. We could not estimate the risk of HNC for the CP patients who were not smokers or drinkers. However, compared with the individuals without chronic pancreatitis and any other comorbidity, the risk of developing HNC was consistently greater in the patients with only CP accompanied by no comorbidity (aHR =2.79, 95% CI: 1.66–4.67). We therefore acknowledge the requirement of more studies to find more co-existing risk factors or comorbidities to recommend a screening program for the CP patients since the NNH was greater than 300 based on our findings. Second, the NHIRD lacked information on human papillomavirus infection and the tumor-staging for HNC. Furthermore, the asymptomatic or mildly symptomatic CP could be missed diagnoses or under-estimated in NHIRD. For the United States between 1973 and 2003, the annual incidence of smoking-related HNC, mostly arising in the oral cavity, larynx, or hypopharynx, has declined at a rate of 1.85% annually; whereas the annual incidence of HPV-related HNC, frequently arising in the oropharynx, has increased at a rate of 0.8% annually (35,36). By the contrast, the incidence of HNC, either HPV-associated or HPV-unassociated, in Taiwan has increased between 1995–2009 (2). Moreover, it has been estimated that the trend for the increasing incidence of oral and oropharyngeal cancer in males will continue and then start to decline after 2025 and that of hypopharyngeal cancer will reach to plateau after 2030 (37). Anyway, CP was consistently related to the development of HNCs after including or excluding the possible HPV-related HNC although we could not provide the information of HPV infection based on our database. Third, there might be surveillance bias in our study because of more opportunities to access oral cancer screening, medical referrals or visits for the patients with CP to find more HNC. However, CP was consistently related to the development of HNC in multivariable analysis after adding frequency of medical visits to be the confounding factor for adjustment. Fourth, we could not individually validate the coding accuracy, but the government have statutorily audited all insurance claims and diagnosis codes for insurance claims. Moreover, the diagnosis of common chronic diseases and healthcare resource utilization have been reported to be substantially concordant between NHIRD and a survey of patient self-reports (38). Finally, our observational cohort study could not confirm the pathogenesis for the aforementioned association between CP and HNC. We acknowledge that our retrospective study is subject to the deficiency of some potential confounding factors. It needs more studies to ascertain the pathogenesis for the association between CP and HNC. It is hard to separate risk factors for CP and HNC because both of them share several etiologies, particularly for the habits of tobacco smoking and alcohol drinking. However, our findings support the close association between CP and HNC. It is argued about recommending a randomized controlled trial (RCT) on a disease of HPV-unrelated HNC with a declining incidence. However, it still should be considered to conduct a RCT on HPV-related HNC since our findings shows that CP was consistently related to the development of HNCs after including or excluding the possible HPV-related HNC.
In conclusion, our population-based cohort study demonstrated that CP is related to an increased risk of HNC, and the presence of comorbidities will increase the risk. However, it requires more randomly-controlled prospective and clinical trial to ascertain the association and pathogenesis between CP and HNC. Detecting populations at high risk for HNC is important for preventing the development of this cancer, particularly finding more co-existing risk factors or comorbidities to diminish the NNH before recommending a screening program for the CP patients. In addition to functional insufficiency of pancreas, chronic pain, and pancreatic cancer, it should be considered to launch a screening program on HNC for CP patients, particularly for those with comorbidities. We believe our results could help the public to adopt appropriate strategies for HNC prevention.
Strengths and limitations of this study
- Our study with a large sample size and 12-year-long follow-up resulted in high statistical power and low selection bias.
- The present findings provided the generalization in Taiwan since the National Insurance Program covered more than 99.6% of Taiwan’s residents.
- This database had limited information on the patients' lifestyle such as smoking habits and alcohol drinking.
Acknowledgments
Funding: The grants of this study were supported by the Ministry of Health and Welfare, Taiwan (MOHW108-TDU-B-212-133004), China Medical University Hospital; Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 107-2321-B-039-004); Tseng-Lien Lin Foundation, Taichung, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders did not participate in study design, data collection or analysis, or preparation of the manuscript for publication. We did not receive other external funding for this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The NHIRD encrypted all the personal information, such as claims information, including sex, date of birth, medical services received, and prescriptions, by anonymous identification numbers to waive the patient consent for accessing. This study has been approved by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-CR4).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975-2011. National Cancer Institute, 2014.
- Hwang TZ, Hsiao JR, Tsai CR, et al. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995–2009. Int J Cancer 2015;137:395-408. [Crossref] [PubMed]
- Maasland DH, van den Brandt PA, Kremer B, et al. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands Cohort Study. BMC Cancer 2014;14:187. [Crossref] [PubMed]
- Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am 2015;24:379-96. [Crossref] [PubMed]
- Wissinger E, Griebsch I, Lungershausen J, et al. The economic burden of head and neck cancer: A systematic literature review. Pharmacoeconomics 2014;32:865-82. [Crossref] [PubMed]
- Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: Evidence-based report on diagnostic guidelines. Pancreas 2014;43:1143-62. [Crossref] [PubMed]
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252-61. [Crossref] [PubMed]
- Domínguez Muñoz JE. Diagnosis of chronic pancreatitis: Functional testing. Best Pract Res Clin Gastroenterol 2010;24:233-41. [Crossref] [PubMed]
- Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011;106:2192-9. [Crossref] [PubMed]
- Lévy P, Domínguez-Muñoz E, Imrie C, et al. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J 2014;2:345-54. [Crossref] [PubMed]
- Bradley EL 3rd. Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg 1987;153:207-13. [Crossref] [PubMed]
- Amann ST, Yadav D, Barmada MM, et al. Physical and mental quality (QOL) in chronic pancreatitis (CP): A case-control study from the NAPS52 cohort. Pancreas 2013;42:293-300. [Crossref] [PubMed]
- Samarasekera E, Mahammed S, Carlisle S, et al. Pancreatitis: summary of NICE guidance. BMJ 2018;362:k3443. [Crossref] [PubMed]
- Chen CH, Lin CL, Hsu C, et al. A retrospective administrative database analysis of suicide attempts and completed suicide in patients with chronic pancreatitis. Front Psychiatry 2018;9:147. [Crossref] [PubMed]
- Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J 2017;5:153-99. [Crossref] [PubMed]
- Chuang SL, Su William WY, Chen Sam LS, et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017;123:1597-609. [Crossref] [PubMed]
- Database NHIR. Taiwan. Available online: (cited in 2015).http://nhird.nhri.org.tw/en/index.html
- Chen CH, Lin CL, Kao CH. Association between gallbladder stone disease and prostate cancer: A nationwide population-based study. Oncotarget 2016;7:64380-9. [PubMed]
- Chen CH, Lin CL, Kao CH. Gastroesophageal reflux disease with proton pump inhibitor use is associated with an increased risk of osteoporosis: a nationwide population-based analysis. Osteoporos Int 2016;27:2117-26. [Crossref] [PubMed]
- Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int. J. Epidemiol 2000;29:336-43. [Crossref] [PubMed]
- Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol 2015;45:291-6. [Crossref] [PubMed]
- Bang UC, Benfield T, Hyldstrup L, et al. Mortality, cancer, comorbidities associated with chronic pancreatitis: A Danish nationwide matched-cohort study. Gastroenterology 2014;146:989-94. [Crossref] [PubMed]
- Hall TC, Garcea G, Webb MA, et al. The socioeconomic impact of chronic pancreatitis: a systematic review. J Eval Clin Pract 2014;20:203-7. [Crossref] [PubMed]
- Wang LW, Li ZS, Li SD, et al. Prevalence and clinical features of chronic pancreatitis in China. A retrospective multicenter analysis over 10 years. Pancreas 2009;38:248-54. [Crossref] [PubMed]
- Garg PK. Chronic pancreatitis in India and Asia. Curr Gastroenterol Rep 2012;14:118-24. [Crossref] [PubMed]
- Sankaran SJ, Xiao AY, Wu LM, et al. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015;149:1490-1500.e1. [Crossref] [PubMed]
- Chiu HY, Hsieh CF, Chiang YT, et al. The risk of chronic pancreatitis in patients with psoriasis: a population-based cohort study. PLoS One 2016;11:e0160041. [Crossref] [PubMed]
- Hwang E, Johnson-Obaseki S, McDonald JT, et al. Incidence of head and neck cancer and socioeconomic status in Canada from 1992 to 2007. Oral Oncol 2013;49:1072-6. [Crossref] [PubMed]
- Menvielle G, Luce D, Goldberg P, et al. Smoking, alcohol drinking, occupational exposures and social inequalities in hypopharyngeal and laryngeal cancer. Int J Epidemiol 2004;33:799-806. [Crossref] [PubMed]
- Masoudkabir F, Sarrafzadegan N, Gotay C, et al. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017;263:343-51. [Crossref] [PubMed]
- Tseng KS, Lin C, Lin YS, et al. Risk of head and neck cancer in patients with diabetes mellitus. JAMA Otolaryngol Head Neck Surg 2014;140:746-53. [Crossref] [PubMed]
- Li S, Lee YC, Li Q, et al. Oral lesions, chronic diseases and the risk of head and neck cancer. Oral Oncol 2015;51:1082-7. [Crossref] [PubMed]
- Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev 2009;18:541-50. [Crossref] [PubMed]
- Wu Y, Antony S, Meitzler JL, et al. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 2014;345:164-73. [Crossref] [PubMed]
- Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: An emerging epidemic of human papillomavirus-associated cancers? Cancer 2007;110:1429-35. [Crossref] [PubMed]
- Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612-9. [Crossref] [PubMed]
- Hsu WL, Yu KJ, Chiang CJ, et al. Head and Neck Cancer Incidence Trends in Taiwan, 1980~2014. Int J Head Neck Sci 2017;1:180-9.
- Wu CS, Lai MS, Gau SSF, et al. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One 2014;9:e112257. [Crossref] [PubMed]


