
Rationale and design of a prospective multi-center randomized trial of EARLY treatment by rivaroxaban versus warfarin in ST-segment elevation MYOcardial infarction with Left Ventricular Thrombus (EARLY-MYO-LVT trial)
Introduction
Despite advances in reperfusion and adjunctive medical therapy, complications of ST-segment elevation myocardial infarction (STEMI) remain crucial causes of worse prognosis (1,2). The formation of left ventricular thrombus (LVT) subsequent to STEMI is not rare, with an incidence as high as 31–57% when thrombolysis is used as the main method of reperfusion (3-5). In the era of mechanical reperfusion, the risk of LVT is lower, but can still be around 15% (6). The existence of LVT is clearly associated with increased risks of embolic events and mortality (7-9). In a meta-analysis published in 2019, the rate of embolic events in STEMI patients with LVT was 3.97 times higher (95% CI, 2.68–5.89) than in those without LVT (10).
Current STEMI guidelines recommend additional anticoagulation on the basis of antiplatelet treatment in patients developing LVT, with vitamin K antagonist (VKA) as the standard anticoagulant agent. The 2013 ACC/AHA guideline for STEMI management suggested adding VKA to a dual antiplatelet therapy (DAPT) in patients with LVT for at least 3 months (11). Similarly, the 2014 American Stroke Association (ASA) guideline for primary prevention of stroke gives a IIa recommendation for using VKA adjunctive to DAPT in STEMI patients with LVT (12). The addition of VKA seems effective in both resolving LVT and reducing embolic events (13,14). However, it is restricted by the requirement of frequent monitoring of international normalized ratios (INRs), especially for patients who have difficulty in accessing medical services regularly, and by INR variability resulting in either decreased effects or increasing risk of bleeding.
Non-VKA oral anticoagulants (NOACs) have shown confirmed advantages compared with VKA in the prevention of embolic events derived from the thrombus in venous system. Nevertheless, their roles in the treatment of LVT remain unclear. Recently, NOACs have been reported in several case reports to effectively resolve post-STEMI LVT together with DAPT, suggesting they could also be promising alternatives to VKA in this clinical scenario.
Therefore, the EARLY-MYO-LVT study has been designed to systemically evaluate the efficacy and safety of rivaroxaban, a potent Xa factor inhibitor, in comparison to VKA in STEMI patients who are in the early phase of LVT after symptom onset.
Methods
Study design and setting
The EARLY-MYO-LVT trial (Clinical trial number: NCT03764241) will be a prospective, multicenter, randomized, open-label, parallel-group, noninferiority trial to evaluate the efficacy and safety of rivaroxaban- versus VKA-based triple antithrombotic therapy in the treatment of early developed LVT after STEMI.
Patients with STEMI will undergo cardiac imaging examinations within 1st month. Those developing LVT will be treated with different antithrombotic regimens for 3 months, during which patients will receive repeated imaging examinations and clinical follow-up. The trial will enroll 280 patients at 10 centers in China. The patient safety and scientific conduct in this study will be supervised by an independent Data and Safety Monitoring Board (DSMB). The flowchart of the trial is illustrated in Figure 1.
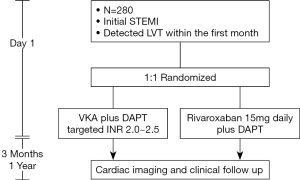
Patient population
Consecutive patients aged 18–75 years who undergo first-time STEMI and develop LVT in the first month of symptom onset are eligible for study participation. Major exclusion criteria are contraindications to anticoagulation and current anticoagulant therapy. Complete inclusion and exclusion criteria are listed in Table 1.

Full table
Randomization
The enrolled patients will be assigned to rivaroxaban or VKA group at 1:1 ratio in 24 hours after the confirmation of LVT. A central computer-generated randomization will be used for all participant centers.
Study treatments
All patients will start DAPT therapy right after the diagnosis of STEMI according to the current guidelines (11,15). Enrolled patients will start dedicated treatments for LVT on the same day of randomization. Patients assigned to the rivaroxaban (Bayer Co. XARELTO) group will take 15 mg daily rivaroxaban on the basis of 100 mg daily aspirin and 75 mg daily clopidogrel. Patients assigned to the VKA group will initially take 5 mg daily warfarin in addition to the same regimen of DAPT. Because VKA initially increases the risk of thrombosis due to temporarily inhibit protein C, S and Z (16), low molecular weight heparin will be overlapped at least five days until the dose of warfarin is titrated to maintain an INR value in the 2–2.5 range (17). In the case of patients on non-clopidogrel P2Y12 antagonists before randomization, the medication will be changed to clopidogrel with a loading dose of 600 mg when the triple therapy starts (18). Replacement of clopidogrel by other P2Y12 antagonists is not allowed during the study period. To prevent gastrointestinal (GI) bleeding, proton pump inhibitor (PPI) use is mandatory for 1 year for all patients. All other concomitant medications will be administrated according to the current STEMI guidelines.
Triple antithrombotic therapy will continue for 3 months in both groups. A forgotten dose of both rivaroxaban and warfarin could be taken up within 12 h of scheduled intake. Otherwise, dose should be skipped and the next scheduled dose should be taken (19). Patients are required to report any interruption of treatments due to personal reasons.
Patients education will be conducted at enrollment to enhance adherence. A day-marked medication box will be delivered to each participant.
Treatment may be discontinued immediately for emergent situations including major bleeding events or urgent invasive procedures. Other situations that may lead to suspension of treatments should be evaluated and determined by the DSMB. Major bleeding will be treated according to the latest practical guide (19).
According to the newest guideline, patients with residual thrombus after 3-month therapy will continue triple anticoagulant treatment until thrombus resolution or up to 6 months unless they are at increased risk of bleeding (15). Table 2 shows the timeline of the study.
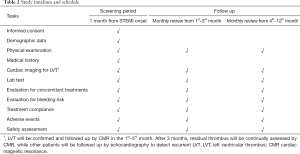
Full table
Study endpoints
Study endpoints are summarized in Table 3. The primary efficacy endpoint is the rate of LVT resolution assessed by cardiac imaging, such as cardiac magnetic resonance imaging (CMR), after 3 months of triple antithrombotic therapy. The incidence of any systemic embolic events and composite adverse events including all-cause death, recurrent myocardial infarct, systemic embolism within 3 months and 1 year after triple therapy will also be compared between the two regimens.

Full table
The primary safety endpoint is the incidence of major bleeding events during triple therapy. A major bleeding is defined as any of the following situations according to the International Society on Thrombosis and Hemostasis (ISTH) criteria: (I) A ≥20 g/L fall in hemoglobin. (II) A transfusion of ≥2 units of red blood cells or whole blood. (III) Bleeding at critical sites including intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome and retroperitoneal. (IV) Bleeding with a fatal outcome (20,21). The incidence of other non-major bleeding events will also be recorded.
Study procedures
During the screening period (day 1 to 30), patients will undergo echocardiography examination at days 7, 14 and 30. Exclusion of LVT during this period will be determined by imaging experts at each center. Suspicious LVT on echocardiography will be confirmed by CMR in 24 hours. An example of manifestations of LVT by CMR is shown in Figure 2. During the treatments, CMR will be repeated at day 30, 60 and 90 (±2 days) to monitor the change of thrombus. All CMR images will be sent to a core lab for off-line analysis as we previously described (1). An independent, double-blinded imaging panel will adjudicate the occurrence and resolution of LVT centrally.
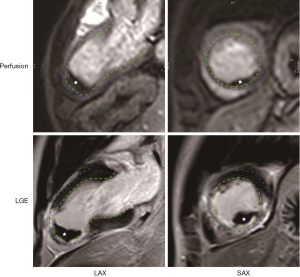
A routine clinical follow-up will be performed each month for 1 year after randomization. Clinical status, including activity tolerance, adverse events, and medical compliance will be documented. Participants and family members are required to report major bleeding and MACEs as soon as possible. Patients assigned to the warfarin group are required to undergo INR testing once every two weeks to measure time in therapeutic range (TTR) during the treatment. Dose adjustment of warfarin will be performed if necessary.
Study data will be documented by certificated medical assistants under DSMB and clinical research associate (CRA) monitoring regulation.
Sample size and statistical analysis
The sample size of the trial was calculated based on the rate of LVT resolution. There was no clinical trial specifically designed to compare the effect of warfarin-based triple therapy versus placebo or other regimen on LVT resolution. Two recent studies mentioned that warfarin-based triple therapy resolved 87.5% and 92.3% of LVT, respectively (13,14). According to the China Clinical Trial Statistics Working Group (CTTS) guideline (22) and the International Council for Harmonisation (ICH) 10 principle (23), an 85–90% preservation of the efficacy of the control agent is reasonable for the non-inferiority test in this situation. Thus, the non-inferiority margin for the rate of LVT resolution by rivaroxaban was set at 76.1% for this trial. With a reported 81–88% of LVT resolution by rivaroxaban-based antithrombotic therapy in case reports, (14,24,25) and an estimated 8% lost of follow-up, the trial will require 280 patients (140 in both arms) to conclude the noninferiority of rivaroxaban to warfarin at a one-side α=0.025 level with 80% power. The sample size was calculated by using the PASS software version 15 (NCSS LLC, USA).
Statistical analysis will be conducted based on intention-to-treat analysis. Continuous data will be presented as the mean ± standard deviation or the median with interquartile range. Categorical data will be presented as counts and percentage. The rate of LVT resolution will be compared using the Chi-square test. Time to LVT resolution and adverse events will be expressed by Kaplan-Meier curves and compared by the log-rank test. If the noninferiority of rivaroxaban were observed, a superiority test would be explored. All P values will be two-sided, and the statistical significance will be set at 5% level. The proportion and 95% confidence interval (CI) will be calculated. Analyses will be performed using SAS software version 9.3 (SAS Institute, USA).
Discussion
The EARLY-MYO-LVT trial aims to be the first randomized clinical trial to evaluate the efficacy and safety of rivaroxaban compared with warfarin in the treatment of early developed LVT after STEMI. The hypothesis is that rivaroxaban will be non-inferior to VKA when used in conjunction with standard DAPT from the perspectives of both the resolution of LVT and the risk of bleeding events.
In a study including 2,911 patients, 93.2% of post-STEMI LVT occurred subsequent to the occlusion of left artery descending (LAD). Large infarction extent, low left ventricular ejection fraction and the formation of ventricular aneurysm are risk factors of LVT (26). LVT greatly increases the incidence of adverse events including systemic embolism and death. The rate of 5-year embolic events could be up to 16.9% in STEMI patients with LVT who did not receive effective anticoagulant therapy, significantly higher than a rate of 2.9% in patients without LVT (27).
An additional anticoagulant agent is thus essential on the basis of antiplatelet therapy as recommended by the current guidelines (11,12,15). Because the majority of LVT is detected within the first month after STEMI onset (14,28), most patients need combined antithrombotic therapy including DAPT and one anticoagulant agent. Early-developed LVT can be effectively resolved by VKA-based triple therapy, and the incidence of systemic embolism can be reduced to nearly 3% (27). Besides their preventive effects on thrombus formation, NOACs also have been used to resolve existing thrombus in the venous system and atrium. For example, in the X-TRA study, which explored the efficacy of rivaroxaban on the resolution of left atrial thrombus in patients with atrial fibrillation, 15 mg daily rivaroxaban resolved 41% of thrombus. However, current practice using rivaroxaban as well as other NOACs to resolve LVT is empirical. A recent systemic review reported that the rate of LVT resolution by NOACs in case reports was around 81–100% (29). Table 4 summarizes 21 case reports using rivaroxaban to treat post-MI LVT. It is suggested from these cases that ≥15 mg daily rivaroxaban is effective to resolve LVT on the basis of antiplatelet agents.
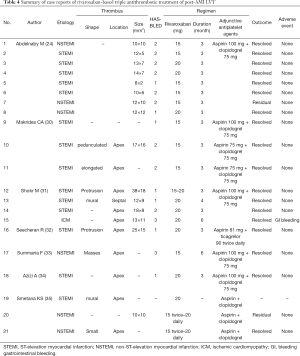
Full table
VKA-based triple antithombotic therapy has been associated with increasing risk of bleeding events. Dr. Maniwa once reported a 2.17% rate of major bleeding in the warfarin-based triple antithrombotic treatment of LVT (27). The incidence was even higher in other larger studies using warfarin-based triple regimen. For example, the WOEST study reported a 5.6% rate of major bleeding (36) and the ISAR-TRIPLE trial reported a 5.3% rate of major bleeding (37). Similarly, although the combination of low-dose rivaroxaban with DAPT has been demonstrated to be safe in STEMI patients with atrial fibrillation by the PIONEER-AF study (38), the ATLAS ACS-TIMI 46 study, in which 5, 10, 15, 20 mg daily rivaroxaban was respectively combined with DAPT in ACS patients with high ischemic risk, showed an apparent dose-dependent bleeding risk. Therefore, 15 mg daily rivaroxaban will be used as the treating dosage in this study to achieve a reasonable efficacy/bleeding risk balance, which was associated with an acceptable 1.79% rate of TIMI major bleeding events in the ATLAS ACS-TIMI 46 study (39).
Currently, echocardiography remains the routine modality to detect LVT in practice. However, recent studies have demonstrated the advantages of CMR compared to echocardiography in visualizing and evaluating LVT (40). Moreover, CMR is the reference method for the volumetric and necrotic assessment of LV. Therefore, CMR will be employed as the confirmatory modality in this trial to achieve more precise detection and follow-up of LVT, as well as functional evaluation of LV.
Rivaroxaban has showed superiority to warfarin in different clinical scenarios that require anticoagulant treatments (25,29,38,39,41-43). Accordingly, the safety and efficacy of rivaroxaban to treat LVT would also be reasonably expected. In the 2017 ESC guidelines for STEMI management, the choice of anticoagulation in patients with LVT is not literally limited to VKA by the first time (15). However, to the best of our best knowledge, there has been no RCT evidence on NOACs versus warfarin in patients with STEMI-related LVT. This trial will use the rate of LVT resolution as the primary efficacy outcome. It is logically conceivable that the resolution of LVT will tightly correlate with the reduction of embolic events. Therefore, the result of the study will provide a solid basis to inform larger RCTs using clinical outcomes as primary endpoints if the hypothesis is proven.
Acknowledgments
We are grateful to Ms. Hu-Wen Wang for her great help in the statistical design.
Funding: This work is supported by National Key Research and Development Program of China (2018YFC1312802), National Science Fund for Distinguished Young Scholars [81625002], National Natural Science Fundation of China [81930007, 81770238], Shanghai Outstanding Academic Leaders Program (18XD1402400), Shanghai Shen Kang Hospital Development Center (16CR3034A), Shanghai Health and Family Planning commission (20184Y0022), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant [20152209], Shanghai Jiao Tong University School of Medicine (DLY201804 and YG2016MS45) and Clinical Research of Renji hospital (PY2018-III-06).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The design and conduct of the study are in accordance with the Declaration of Helsinki and Chinese regulations. Ethics committee of Renji Hospital has approved the study protocol on June 24. 2019 (Approval number: KY-2019-057). All participants will sign written informed consent before randomization.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pu J, Ding S, Ge H, et al. Efficacy and Safety of a Pharmaco-Invasive Strategy With Half-Dose Alteplase Versus Primary Angioplasty in ST-Segment-Elevation Myocardial Infarction: EARLY-MYO Trial (Early Routine Catheterization After Alteplase Fibrinolysis Versus Primary PCI in Acute ST-Segment-Elevation Myocardial Infarction). Circulation 2017;136:1462-73. [Crossref] [PubMed]
- Ding S, Lin N, Sheng X, et al. Melatonin stabilizes rupture‐prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα‐dependent manner. J Pineal Res 2019;67:e12581. [Crossref] [PubMed]
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990;15:790-800. [Crossref] [PubMed]
- Mooe T, Teien D, Karp K, et al. Left ventricular thrombosis after anterior myocardial infarction with and without thrombolytic treatment. J Intern Med 1995;237:563-9. [Crossref] [PubMed]
- Domenicucci S, Chiarella F, Bellotti P, et al. Early appearance of left ventricular thrombi after anterior myocardial infarction: a marker of higher in-hospital mortality in patients not treated with antithrombotic drugs. Eur Heart J 1990;11:51-8. [Crossref] [PubMed]
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010;106:1197-200. [Crossref] [PubMed]
- Kouvaras G, Chronopoulos G, Soufras G, et al. The effects of long-term antithrombotic treatment on left ventricular thrombi in patients after an acute myocardial infarction. Am Heart J 1990;119:73-8. [Crossref] [PubMed]
- Cregler LL. Antithrombotic therapy in left ventricular thrombosis and systemic embolism. Am Heart J 1992;123:1110-4. [Crossref] [PubMed]
- Ram P, Shah M, Sirinvaravong N, et al. Left ventricular thrombosis in acute anterior myocardial infarction: Evaluation of hospital mortality, thromboembolism, and bleeding. Clin Cardiol 2018;41:1289-96. [Crossref] [PubMed]
- Chen PF, Tang L, Yi JL, et al. The prognostic effect of left ventricular thrombus formation after acute myocardial infarction in the contemporary era of primary percutaneous coronary intervention: A meta-analysis. Eur J Intern Med 2019. [Epub ahead of print]. [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362-425. [PubMed]
- Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45):3754-832.
- Delewi R, Nijveldt R, Hirsch A, et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol 2012;81:3900-4. [Crossref] [PubMed]
- Meurin P, Brandao Carreira V, Dumaine R, et al. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low left ventricular ejection fraction: a prospective multicenter study. Am Heart J 2015;170:256-62. [Crossref] [PubMed]
- Kristensen SD, Aboyans V. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e44S-88S.
- Lip GYH, Collet JP, Haude M, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019;21:192-3. [Crossref] [PubMed]
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60. [Crossref] [PubMed]
- Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330-93. [Crossref] [PubMed]
- Schulman S, Angeras U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202-4. [Crossref] [PubMed]
- Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119-26. [Crossref] [PubMed]
- Xia JL. Statistical considerations in non-inferiority clinical trials. Chinese Journal of Health Statistics 2012;29:270-74.
- ICH Harmonized Tripartite Guideline. Choice of control group and related issues in clinical trials. 2000;E10.
- Abdelnaby M, Almaghraby A, Abdelkarim O, et al. The role of rivaroxaban in left ventricular thrombi. Anatol J Cardiol 2019;21:47-50. [PubMed]
- Leow AS, Sia CH, Tan BY, et al. A meta-summary of case reports of non-vitamin K antagonist oral anticoagulant use in patients with left ventricular thrombus. J Thromb Thrombolysis 2018;46:68-73. [Crossref] [PubMed]
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008;335:171-6. [Crossref] [PubMed]
- Maniwa N, Fujino M, Nakai M, et al. Anticoagulation combined with antiplatelet therapy in patients with left ventricular thrombus after first acute myocardial infarction. Eur Heart J 2018;39:201-8. [Crossref] [PubMed]
- Küpper AJF, Verheugt FW, Peels CH, et al. Left ventricular thrombus incidence and behavior studied by serial two-dimensional echocardiography in acute anterior myocardial infarction: left ventricular wall motion, systemic embolism and oral anticoagulation. J Am Coll Cardiol 1989;13:1514-20. [Crossref] [PubMed]
- Kajy M, Shokr M, Ramappa P.. Use of Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus: Systematic Review of Current Literature. Am J Ther 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Makrides CA. Resolution of left ventricular postinfarction thrombi in patients undergoing percutaneous coronary intervention using rivaroxaban in addition to dual antiplatelet therapy. BMJ Case Rep 2016. [Crossref] [PubMed]
- Shokr M, Ahmed A, Abubakar H, et al. Use of direct oral anticoagulants in the treatment of left ventricular thrombi: A tertiary center experience and review of the literature. Clin Case Rep 2018;7:135-42. [Crossref] [PubMed]
- Seecheran R, Seecheran V, Persad S, et al. Rivaroxaban as an antithrombotic agent in a patient with ST-segment elevation myocardial infarction and left ventricular thrombus: a case report. J Investig Med High Impact Case Rep 2017;5:2324709617697991. [Crossref] [PubMed]
- Summaria F, Sgueglia GA, D'Errico F, et al. Safety and Efficacy of Triple Therapeutic Targets with Rivaroxaban after Acute Myocardial Infarction Complicated by Left Ventricular Thrombi in a Case of Nonvalvular Atrial Fibrillation. Case Rep Cardiol 2018;2018:6503435.
- Azizi A, Puricel S, Cook S, et al. Rivaroxaban dissolves postinfarction left ventricular thrombus. Cardiovasc Med 2016;19:25-7. [Crossref]
- Smetana KS, Dunne J, Parrott K, et al. Oral factor Xa inhibitors for the treatment of left ventricular thrombus: a case series. J Thromb Thrombolysis 2017;44:519-24. [Crossref] [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- Fiedler KA, Maeng M, Mehilli J, et al. Duration of Triple Therapy in Patients Requiring Oral Anticoagulation After Drug-Eluting Stent Implantation: The ISAR-TRIPLE Trial. J Am Coll Cardiol 2015;65:1619-29. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 2009;374:29-38. [Crossref] [PubMed]
- McCarthy CP, Vaduganathan M, McCarthy KJ, et al. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol 2018;3:642-9. [Crossref] [PubMed]
- Mega JL, Braunwald E, Murphy SA, et al. Rivaroxaban in patients stabilized after a ST-segment elevation myocardial infarction: results from the ATLAS ACS-2-TIMI-51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction-51). J Am Coll Cardiol 2013;61:1853-9. [Crossref] [PubMed]
- Lip GY, Hammerstingl C, Marin F, et al. Left atrial thrombus resolution in atrial fibrillation or flutter: Results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J 2016;178:126-34. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]


