Comparison between Child-Pugh Score and albumin-bilirubin grade in patients treated with the combination therapy of transarterial chemoembolization and sorafenib for hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death; for the coexistence of underlying liver disease, the prognosis assessments of the HCC patients remain complicated and should take the tumor burden, liver function and physical status into consideration (1,2). Generally, the Child-Pugh classification is routinely used to evaluate the liver function in HCC patients and even plays an important role in most of the prevalent staging systems (3). However, the Child-Pugh classification is originally proposed for the patients with cirrhosis and portal hypertension undergoing surgery for variceal bleeding rather than HCC (4,5); moreover, the Child-Pugh classification might introduce subjectivity for including the factors of ascites and encephalopathy (6). Recently, a new model for the evaluation of liver function only according to albumin and bilirubin (ALBI) has shown high discriminating value in predicting survival for patients with HCC and has been widely validated all over the world (7,8).
According to the BCLC staging system, transarterial chemoembolization (TACE) is the standard therapy for intermediate HCC, while the systemic therapy is recommended to those with advanced disease (2,9). Sorafenib, as the first approved systemic treatment, is usually combined with TACE for unresectable HCC in clinical practice (10-12). Several prospective cohort studies have demonstrated the safety and effectiveness of this novel treatment modality (13,14). In this study, we aimed to compare the prognostic value of ALBI score and Child-Pugh score in HCC patients with Child-Pugh A who had received the combination treatment of TACE and sorafenib.
Methods
Study population
Study data were extracted from a database of 1,487 consecutive HCC patients treated at our center from January 2010 to June 2014. The HCC was diagnosed according to American Association for the Study of the Liver Disease or European Association for the Study of Liver disease (AASLD/EASL) guidelines (9,15). Patients with Child-Pugh A classification who were being treated with the combination therapy of TACE and sorafenib were included; meanwhile, patients meeting one of the following criteria were excluded: the presence of extrahepatic spread (EHS), Eastern Cooperative Oncology Group (ECOG) performance status >1 and decompensated liver function with ascites, jaundice or encephalopathy. Written informed consent was obtained from all patients before the administration of TACE or sorafenib; and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board.
Treatment and follow-up
Before the TACE, hepatic artery angiography was carried out to evaluate the vascular anatomy and tumor vascularity; after that, a vascular catheter was inserted selectively into the tumor-feeding artery with an injection containing a mixture of doxorubicin (10–50 mg) and lipiodol (2–20 mL), followed by an embolization using gelatin sponge particles. Sorafenib was continuously administered for thirty days before or after first TACE, with an initial dose of 400 mg twice daily. Laboratory assessments were conducted every four to six weeks; imaging evaluation was performed during the fourth and eighth weeks after treatment and every eight weeks thereafter through the contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). When residual viable tumors were confirmed or new lesions developed in patients with adequate liver function, repeated TACE was allowed. Patients were always encouraged to continue sorafenib administration, unless unmanageable or life-threatening adverse events occurred. According to the presence of toxicities, sorafenib dose modification was permitted. Adverse events were assessed by three independent physicians using the Common Terminology Criteria for Adverse Events (CTCAE; version 3.0) from the National Cancer Institute of China.
Statistical analysis
Categorical variables were summarized by frequencies and percentages; continuous variables were described by average with standard deviation (SD) or median with interquartile range (IQR). Overall survival (OS) was defined as the time from the first session of TACE until death or until last follow-up; and the survival analysis was conducted using Kaplan-Meier method and compared with log-rank test. Cox-proportional hazard regression models were used to estimate hazard ratios for risk factors in relation to OS. Three multivariate models with stepwise methods were separately conducted for selecting the independent prognostic factors: model 1 including the baseline characteristics; model 2 including the baseline characteristics and Child-Pugh score but excluding albumin and bilirubin; model 3 including the baseline characteristics and ALBI score but also excluding albumin and bilirubin. To compare the discriminatory abilities of Child-Pugh score and ALBI score for OS, the time-dependent receiver operating characteristic (ROC) curve was used at different follow-up time points. Finally, the survival analyses according to Child-Pugh score and ALBI SCORE were separately repeated for multiple subgroups of patients with different baseline backgrounds. Statistical analyses were conducted using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
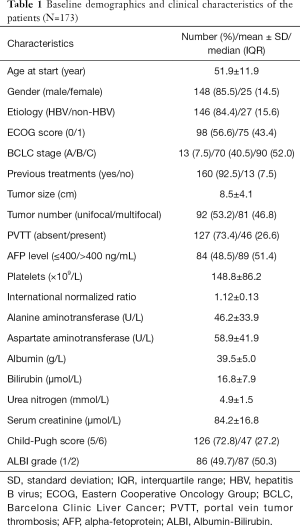
According to the protocol, this study cohort consisted of 173 patients with a mean age of 51.9 years. Of the enrolled patients, 148 (85.5%) patients were male and 146 (84.4%) patients had HBV infection. According to the BCLC staging system, 13 (7.5%), 70 (40.5%) and 90 (52.0%) patients belonged to the stage of A, B and C, respectively. Ninety-eight (56.6%) patients had no tumor-related symptoms and the majority of the patients (160, 92.5%) had received no previous treatment. The mean tumor size (maximum diameter of the largest tumor) was 8.5 cm, and 92 (53.2%) patients had a unifocal tumor; in addition, there were 127 (73.4%) patients without portal vein tumor thrombosis. The laboratory examinations at baseline were also summarized in Table 1.
Full table
Survival analyses according to Child-Pugh Score and ALBI
During a mean follow-up of 22.6 months, 145 patients had died and the median OS reached 15.1 [95% confidence interval (CI) 12.3–17.9] months. According to the Child-Pugh system for the evaluation of liver function, 126 (72.8%) and 47 (27.2%) patients had a Child-Pugh score of 5 and 6, respectively. Based on the Kaplan-Meier curves, the patients with a Child-Pugh score of 5 had a longer OS than those with a Child-Pugh score of 6 (16.7 vs. 10.5 months, Log-rank P=0.004; Figure 1A). However, according to the ALBI system, 86 (49.7%) patients belonged to grade 1 and 87 (50.3%) to grade 2 with a mean score of −2.95 and −2.20, respectively. The patients with ALBI grade 1 reached a median OS of 19.0 months, which was better than the patients with ALBI grade 2, who had a median OS of 11.7 months (Log-rank P<0.001; Figure 1B).

Univariate and multivariate analyses
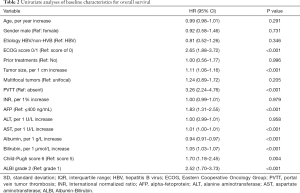
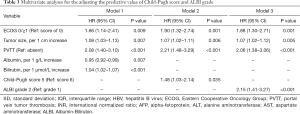
According to the univariate analysis for OS, the ECOG performance status, tumor size, presence of PVTT, AFP level, AST, albumin and bilirubin, as well as Child-Pugh score and ALBI grade were significant prognostic factors (P<0.05; Table 2). In multivariate model 1, ECOG performance status (HR 1.66, P=0.009), tumor size (HR 1.08, P=0.007), presence of PVTT (HR 2.08, P<0.001), albumin (HR 0.95, P=0.007) and bilirubin (HR 1.04, P<0.001) were identified as independent predictors of OS. According to multivariate model 2, the independent prognostic factors included ECOG performance status (HR =1.90, P=0.001), tumor size (HR =1.07, P=0.006), presence of PVTT (HR =2.21, P<0.001) and Child-Pugh score (HR =1.48, P=0.035). For multivariate model 3, the independent predictors of OS were ECOG performance status (HR =1.88, P=0.001), tumor size (HR =1.07, P=0.005), presence of PVTT (HR =2.06, P<0.001) and ALBI grade (HR =2.15, P<0.001) (Table 3). Therefore, according to the multivariate analysis, the Child-Pugh score and ALBI grade could independently predict OS in patients treated with the combination therapy of TACE and sorafenib.
Full table
Full table
Comparison of the discriminatory abilities for Child-Pugh Score and ALBI grade in predicting survival
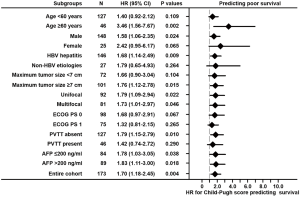
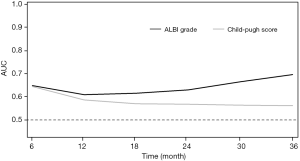
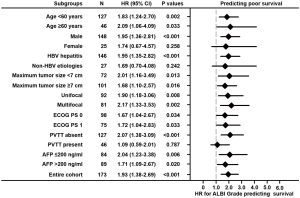
According to the time-dependent ROC analysis, the ALBI grade had a more discriminatory ability than Child-Pugh in predicting survival, especially at the long-term time points (Figure 2). In addition, their predictive abilities were investigated in subsets of patients with different baseline characteristics. As for the ALBI grade, it was a significant predictor of OS in thirteen of sixteen subsets and lost its predictive value in female patients and those with non-HBV etiologies or PVTT (P>0.05; Figure 3). Nevertheless, the Child-Pugh score remained predictive in only nine of sixteen subsets (P<0.05; Figure 4).
Discussion
According to the BCLC staging system, the survival of patients with HCC is determined by the tumor burden, liver function and physical status (2). The Child-Pugh classification is commonly used in the evaluation of liver function; nevertheless, it has several limitations and introduces subjectivity (6). The ALBI grade, proposed by Johnson et al., is a simple and objective method based only on the albumin and bilirubin which has shown a reliable ability to stratify patients with significantly different prognoses, and even performs well in a different staging system when replacing Child-Pugh classification (7,16-18). Especially, for patients with preserved liver function (Child-Pugh A), the ALBI grade performs well in predicting survival (19). Although the ALBI system has been validated in HCC patients treated by curative therapy, locoregional treatment and sorafenib, its discriminatory value in those patients receiving the combination therapy of TACE and sorafenib has never been evaluated (20-22).
According to our study, the ALBI grade and Child-Pugh score can separately predict the survival of HCC patients with Child-Pugh A who are receiving the combination therapy of TACE and sorafenib. When compared to patients with ALBI grade 1, the risk of death increases by 115% for patients with ALBI grade 2, while a Child-Pugh score of 6 increases the risk of death by 48% compared to a Child-Pugh score of 5. This means that the ALBI grade stratifies the patients with different prognosis more obviously and significantly than the Child-Pugh score for those patients with preserved liver function (Child-Pugh A). These findings are consistent with the previous perspectives of the replacement of Child-Pugh Score with ALBI grade as trial inclusion criteria for the participants with preserved liver function (19,23).
Additionally, the ALBI grade was better than Child-Pugh score in terms of the discriminatory ability for OS based on the time-dependent ROC analyses, especially in long-term outcomes. Previous studies have demonstrated that the ALBI grade was comparable to Child-Pugh score for evaluating liver function; however, none of them have investigated and compared their discriminatory values at different time points. Considering the long-term discriminating ability, the ALBI grade might be better than Child-Pugh score in prognostic stratification for patients with preserved liver function (Child-Pugh A). For intermediate HCC, there are great heterogeneity regarding patient characteristics (24). The substage of BCLC B stage has been proposed and widely validated; however, it uses the Child-Pugh score for the assessment of liver function (24,25). Considering that the majority of the patients with intermediate HCC have preserved liver function, it might be more appropriate to use ALBI grade to stratify the intermediate HCC rather than using Child-Pugh score. Recently, Hiraoka et al. developed a new sub-grouping for intermediate-stage HCC based on ALBI grade and found it better than the traditional substage proposed by Bolondi et al. for the evaluation of liver function (26).
Our study for the first time evaluated the performance of the ALBI grade when used to predict survival in HCC patients treated with the combination therapy of TACE and sorafenib and found that the ALBI score might outperform Child-Pugh score in patients with preserved liver function (Child-Pugh A). However, there were some limitations for this study. Firstly, the single-center and retrospective nature of this study might introduce some bias; yet quality control was ensured because all procedures and administrations were conducted by the same experienced team. Secondly, regardless of the performance of the ALBI grade or Child-Pugh score, their discriminating ability disappeared in some of the subsets of patients, which may be due to the small sample size of these subgroups. However, the aim of subgroup analysis was to compare the consistency of the two kinds of scoring systems and it found the ALBI grade was more suitable for the majority of subgroups of patients than Child-Pugh score. Finally, all patients in our study were Chinese, thus the generalization of our findings should be cautious and future studies are needed.
In sum, for HCC patients with preserved liver function (Child-Pugh A) and treated by the combination therapy of TACE and sorafenib, our study demonstrates that the ALBI grade might be better than Child-Pugh score in terms of stratifying prognosis. These observations may have major implications for the future study design.
Acknowledgments
Funding: This study was supported by the Innovation Capability Support Program of Shaanxi province (2018KJXX-076) and the National Natural Science Foundation of China (81702999) for Dr. LL.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.114). LL serves as the unpaid editorial board member of Annals of Translational Medicine from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all patients before the administration of TACE or sorafenib; and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Liu PH, Hsu CY, Hsia CY, et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol 2016;64:601-608. [Crossref] [PubMed]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85. [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis 2008;28:110-22. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Sacco R, Antonucci M, Bargellini I, et al. Transarterial chemoembolization and sorafenib in patients with intermediate-stage hepatocellular carcinoma: time to enter routine clinical practice? Future Oncol 2015;11:2371-3. [Crossref] [PubMed]
- Geschwind JF, Kudo M, Marrero JA, et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology 2016;279:630-40. [Crossref] [PubMed]
- Abou-Alfa GK. TACE and sorafenib: a good marriage? J Clin Oncol 2011;29:3949-52. [Crossref] [PubMed]
- Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol 2012;56:1336-42. [Crossref] [PubMed]
- Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer 2015;136:1458-67. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Chan AW, Chong CC, Mo FK, et al. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1766-72. [Crossref] [PubMed]
- Chan AW, Kumada T, Toyoda H, et al. Integration of albumin-bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1300-6. [Crossref] [PubMed]
- Chan AW, Chong CC, Mo FK, et al. Incorporating albumin-bilirubin grade into the cancer of the liver Italian program system for hepatocellular carcinoma. J Gastroenterol Hepatol 2017;32:221-8. [Crossref] [PubMed]
- Knox JJ. Addressing the interplay of liver disease and hepatocellular carcinoma on patient survival: the ALBI scoring model. J Clin Oncol 2015;33:529-31. [Crossref] [PubMed]
- Toyoda H, Lai PB, O'Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744-50. [Crossref] [PubMed]
- Kao WY, Su CW, Chiou YY, et al. Hepatocellular Carcinoma: Nomograms Based on the Albumin-Bilirubin Grade to Assess the Outcomes of Radiofrequency Ablation. Radiology 2017;285:670-80. [Crossref] [PubMed]
- Ogasawara S, Chiba T, Ooka Y, et al. Liver function assessment according to the Albumin-Bilirubin (ALBI) grade in sorafenib-treated patients with advanced hepatocellular carcinoma. Invest New Drugs 2015;33:1257-62. [Crossref] [PubMed]
- Edeline J, Blanc JF, Johnson P, et al. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. Liver Int 2016;36:1821-8. [Crossref] [PubMed]
- Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012;32:348-59. [PubMed]
- Giannini EG, Moscatelli A, Pellegatta G, et al. Application of the Intermediate-Stage Subclassification to Patients With Untreated Hepatocellular Carcinoma. Am J Gastroenterol 2016;111:70-7. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Nouso K, et al. Proposed New Sub-Grouping for Intermediate-Stage Hepatocellular Carcinoma Using Albumin-Bilirubin Grade. Oncology 2016;91:153-61. [Crossref] [PubMed]