Intracardiac and intravascular leiomyomatosis associated with a pelvic arterio-venous fistula
An intravenous leiomyomatosis is an uncommon benign smooth muscle tumor originating from the uterine or pelvic veins. It is reported that more than a half of the patients have a history of previous hysterectomy. This leiomyomatosis occasionally extends into the right cardiac chambers via the inferior vena cava (IVC) and causes congestive heart failure due to tricuspid stenosis (1). Complete surgical resection is considered the best treatment for leiomyomatosis because recurrence is frequent despite the benign nature of the tumor (2).
Pelvic arterio-venous fistula (AVF) is a rare pelvic vascular malformation that is most commonly congenital, post traumatic or iatrogenic. Massive AV shunting causes high output cardiac failure. This malformation is difficult to eradicate completely because of a high recurrence rate. Surgical resection is often difficult because of severe hemorrhage (3). Transcatheter embolization has recently become the treatment of choice for pelvic AVF, but complete embolization to stop the shut flow is also difficult (4). Preoperative embolization of AVF is now an adjunct strategy to decrease intraoperative blood loss.
We encountered a very rare case of an intracardiac leiomyomatosis extending from a left ovarian vein associated with a pelvic AVF.
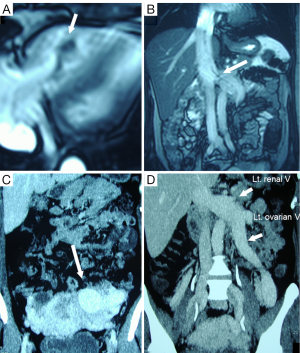
A 48-year-old woman was admitted to our hospital with a 2-month history of dyspnea and leg edema. She underwent a caesarian operation 20 years ago, followed by a bilateral hysterectomy 13 years ago. A chest radiograph showed an enlarged heart (cardio-thoracic ratio: 61%) and lung congestion. Echocardiography and magnetic resonance (MR) images revealed that a large mobile mass originating from the left ovarian vein was floating in the right atrium (RA) and ventricle (RV) (Figure 1A). The intracardiac part was very thick, but the intravascular part in the pelvic veins was very slim (Figure 1B). Enhanced computed tomography (CT) showed a large highly vascular mass around the uterus and left ovary (Figure 1C). The IVC, left renal vein and left ovarian vein were extremely enlarged (Figure 1D). At this time, we were unable to determine the possibility of high-output congestive heart failure caused by the pelvic AVF.
Median sternotomy was performed simultaneously with median laparotomy to expose the heart and IVC, left renal vein, and left ovarian vein. After heparinization, an arterial cannula was inserted into the ascending aorta, and a venous cannula was inserted into the superior vena cava (SVC). Another venous cannula was inserted into the IVC through the right femoral vein. The tip of the venous cannula was positioned below the left renal vein. Cardiopulmonary bypass (CPB) was started, but the heart could not be sufficiently decompressed because of extremely high venous return from the IVC. When a small incision was made at the RA, the saturated blood spouted out from the atrial incision. The thick tip of the mobile tumor was pulled out from the RA and resected. A long suture was fixed at the end of the residual tumor. The tumor was inserted back into the RA, and the atrial incision was quickly closed to stop bleeding. However, the suture attached to the tumor was held out of the atrium to prevent the tumor from going into the pulmonary artery as an embolus. Then, the IVC was clamped just above the IVC cannula, and the dilated left renal vein and left ovarian vein were opened. The saturated venous blood from the ovarian vein was removed with four suction tubes, and the tumor with the suture was pulled out and resected from the left ovarian vein wall, and CPB was stopped.
After neutralization of heparine, the unusual red soft tissue mass in the pelvis was resected with the left ovary and uterus. Massive hemorrhage (more than 8,000 mL) occurred during the resection. A cell-saver system and a rapid blood transfusion system were utilized to maintain the circulation. After resection of the pelvic mass, the heart was relieved of congestion. The pelvic mass consisted of vascular tissue without any tumor tissue. Therefore, we concluded that the pelvic highly vascular mass was associated with a pelvic AVF. The patient recovered without any problem.
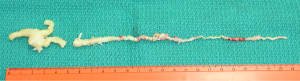
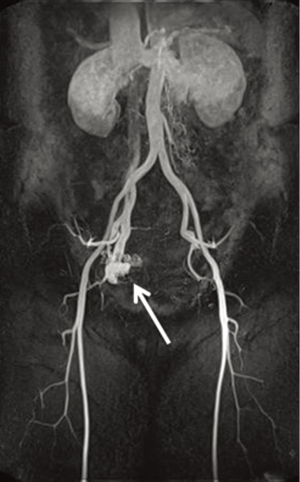
The intracardiac and intravenous tumor was 24 cm long (Figure 2). The intravenous part was 1 to 2 mm in diameter and twisted like a screw, probably due to abnormal high venous blood flow. Pathological study revealed that the tumor was a benign smooth muscle tumor (leiomyomatosis). Post-operative MR angiography showed that the patient also had a pelvic AVF with small shunt at the right side and there was no evidence of recurrence of leiomyomatosis and congestive heart failure 5 years after surgery (Figure 3).
Comments
Lee et al. reported the first case of a pelvic AVF with intravenous leiomyomatosis (5). The patient had congestive heart failure due to massive AV shunting, and the leiomyomatosis was found in the gonadal veins of the resected mass. The present case is the second case of leiomyomatosis associated with a pelvic AVF. However, our patient had a separate pelvic AVF and intracardiac and intravenous leiomyomatosis. Because of the high venous blood flow from the pelvic AVF, the intravenous tumor was extremely twisted and elongated, and the intravenous part was slim. The tumor could have ruptured and induced major pulmonary emboli in the near future. There was no tumor tissue in the resected vascular mass. From this point, the present case is unique.
One of the difficult surgical points in this case is how to control the high venous return from the pelvic AVF at the time of removing the intracardiac and intravenous tumor. It was quite difficult to control the venous return from the left renal vein and the left ovarian vein because a venous cannula could not be inserted into these veins due to the presence of the intravenous tumor. In this case, the venous return from the SVC was controlled by gravity, but the return from the IVC was controlled by an individual pump in the CPB. Two suction tubes are usually utilized in cardiac surgery at our hospital, but in this case, two additional suction tubes were utilized to visualize the tumor. Deep hypothermic circulatory arrest was also considered to be a good surgical option when the venous return could not be controlled.
In conclusion, we encountered a very rare patient who had an intracardiac leiomyomatosis combined with a pelvic AVF. We successfully performed one-stage resection for the leiomyomatosis and the pelvic AVF. We carefully follow up the patient for recurrence of leiomyomatosis and congestive heart failure.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Nili M, Liban E, Levy MJ. Tricuspid stenosis due to intravenous leiomyomatosis--a call for caution: case report and review of the literature. Tex Heart Inst J 1982;9:231-5. [PubMed]
- Ozer N, Engin H, Akgül E, et al. An unusual case of recurrent mass in the right atrium: intravenous leiomyomatosis. Echocardiography 2005;22:514-6. [PubMed]
- McCready RA, Fehrenbacher JW, Divelbiss JL, et al. Surgical resection of a large recurrent pelvic arteriovenous malformation using deep hypothermic circulatory arrest. J Vasc Surg 2004;39:1348-50. [PubMed]
- Jacobowitz GR, Rosen RJ, Rockman CB, et al. Transcatheter embolization of complex pelvic vascular malformations: results and long-term follow-up. J Vasc Surg 2001;33:51-5. [PubMed]
- Lee VS, Thompson NW, Cho KJ, et al. High-output cardiac failure: an unusual manifestation of intravenous leiomyomatosis. Surgery 1993;113:466-70. [PubMed]


