
Effects of estrogen deprivation on memory and expression of related proteins in ovariectomized mice
Introduction
In general, the majority of patients suffering from Alzheimer’s disease (AD) are women, and women have a higher risk of cognitive impairment than men. Although this discrepancy may be related to the longer life span of women, the sharp decline in estrogen during menopause may also contribute to this situation. Hormone replacement therapy has been demonstrated to protect menopausal women against cognitive impairment (1,2), which leads us to consider the relationship between estrogen and memory. To verify the contribution of estrogen to memory, there have been many animal models developed to mimic the state of estrogen deprivation. Generally, the ovariectomized (OVX) rodent model is the most widely used model for this purpose (3). Using this model, many studies have demonstrated the crucial role of estrogen in memory enhancement, although the results have been inconsistent. For instance, Sarkaki et al. found impaired reference memory in OVX rats in the MWM test (4). In contrast, Wilson et al. demonstrated that no significant spatial reference memory deficits were observed in OVX mice in the water maze or the Y-maze tests; however, they did note some working memory deficits in the radial arm maze (5). Although there are many factors that can lead to these discrepancies, such as the age of the animals, the sensitivity of the detection method used and so on, one possible reason is that the duration of estrogen deprivation used in this model varies considerably (6). However, no systematic study has been conducted so far to investigate how memory changes and how the memory-related proteins differ at different time points following OVX surgery.
Brain-derived neurotrophic factor (BDNF) is the most widely studied and expressed neurotrophin in the mammalian brain, especially in cognitive function research. It has been reported that BDNF plays an important role in modulating neuronal differentiation, growth, synaptic plasticity and synaptic efficacy in the development of neuronal circuits (7,8). In fact, many studies have been conducted to investigate the relationship between BDNF and estrogen. Previously, the estrogen response element was considered to be present in the Bdnf genes (9), which suggested that the brain BDNF level might be regulated by estrogen. As expected, epigenetic repression of BDNF was found in the cortex and hippocampus following ovarian deprivation (10), although it could be rescued by estrogen replacement. Given the relationship between BDNF and estrogen, BDNF deficits might be an important cause of impaired memory after OVX (8), while the relationship between the change in BDNF in the process of memory impairment after OVX remains unknown.
Autophagy is defined as a process of sequestering organelles and long-lived proteins in a double-membrane vesicle inside the cell, where the contents are subsequently delivered to lysosomes for degradation (11). Autophagy can be upregulated when cells need to generate intracellular energy and nutrition; it can also be upregulated when cells are undergoing structural remodeling or expelling damaging cytoplasmic components. Obviously, autophagy plays a major role in regulating cellular homeostasis, and it might protect the body from negative effects such as memory dysfunction. In some animal models for AD, the upregulation of autophagy has been reported to reduce the characterized amyloid β and hyperphosphorylated tau, which alleviated memory impairment and pathological phenotypes (12). Moreover, previous studies have suggested that estrogen was related to autophagy (13), though their results were inconsistent. By using quantitative proteomics and Western blot analysis, activation of autophagy was observed in the hippocampus and amygdala of OVX animals (14). In the study of the short-term and long-term effects of estrogen deprivation in the OVX model, autophagy-related proteins showed a gradual decrease. The change in autophagy throughout the process of memory impairment after OVX was not clarified through these studies either (15). Obviously, the progressive change in memory and autophagy-related protein requires further study.
We hypothesized that the memory of the OVX animals would deteriorate in concert with the prolongation of estrogen deprivation and that these memory alterations might correlate with the expression of BDNF and autophagy-related proteins such as Unk-51-like kinase 1 (ULK1) and microtubule-associated protein 1 light chain 3 (LC3). In the present study, we aimed to investigate the progressive effects of estrogen deprivation on the memory of OVX mice and to explore the change in BDNF and ULK1 levels in the hippocampus throughout the development of memory alterations.
Methods
Animals
Female ICR mice (7–8 weeks old, 20–25 g) were purchased from the Beijing Weitong Lihua Experimental Animal Technology Company. All animals were bred and housed five per cage with standard food and water available ad libitum in a humidity- and temperature-controlled room (23±1 °C) with a relative humidity of 60%±5%. The mice were maintained on a 12-h light/dark cycle (lights on at 08:30 am). All animal care and experimental protocols were approved by the Committee on Animal Care and Use of the Institution of Medical Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College.
Experimental design
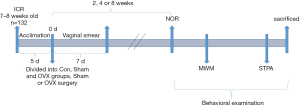
After a 7-day acclimation period, the mice were randomly divided into three groups by body weight: the control group (Con, n=42), the sham-operated group (Sham, n=45) and the OVX group (OVX, n=45). All OVX surgeries were conducted on the same day during the initial phase of the experiment. As shown in Figure 1, at 2, 4 and 8 weeks after OVX surgery, the mice in each group were randomly selected for behavioral examination (n=14, 15 and 15 for the Con, Sham and OVX groups, respectively). The behavioral tests included novel object recognition (NOR), the Morris water maze (MWM) and the step-through passive avoidance (STPA) test, and they were performed successively. Considering the possible influence of circadian rhythm on the behavioral performance of mice, all behavioral detection was conducted from 8:00 am to 14:00 pm. After the behavioral tests, the mice were sacrificed. The brain was quickly removed, and the hippocampus was isolated. The hippocampus was quickly frozen in liquid nitrogen and stored at −80 °C until the Western blot analysis.
OVX surgery
Mice in the Sham and OVX groups were anesthetized with 3.0% pentobarbital sodium (45 mg/kg, intraperitoneal), and some hair on the back was shaved off for surgery. A dorsolateral incision of the skin along the spine was made perpendicular to the line of the base of the thighs. The muscles 0.5 cm beneath the midline of the back beneath the skin were incised, and the fat beneath the muscles was grasped to exteriorize the ovary. The fallopian tube was ligated, and then the ovary was removed by cutting above the ligated area. In the Sham group, the mice underwent the same incisions, and the fallopian tubes and ovaries were exposed but not removed and then put back in the abdominal cavity. Finally, the incisions in the muscle and skin were closed (16).
Vaginal smear examination
The vaginal smear examination was performed during the successive 7 days after surgery. Vaginal contents were collected with a blunted Pasteur pipette by placing a small drop of saline into the vagina; the vaginal cells obtained were immediately observed under a microscope (17). Classified in terms of morphological characteristics, the relative proportion of leukocytes, cornified epithelial cells, and nucleated epithelial cells could be estimated (18). The phases of the estrus cycle could be estimated further according to the differences in cellular appearance. When the leukocytes occupied the majority of the field for at least 4 days, the mice were considered to have lost their estrus cycle, and the ovariectomy was successful.
Behavioral assessment
Novel objection recognition (NOR)
NOR was performed in a box using the methods described by Takuma et al. with a slight modification (19). The box was 60 cm long × 40 cm wide × 40 cm high, and there was a camera connected to a video recording device for recording the animal behavior. First, a mouse was placed in the empty open field once a day for 3 consecutive days (10 min per day) for habituation. Then, 24 h after the last habituation, the training session began. Two identical objects were placed in the corner of the box (approximately 10 cm from the walls), and the mouse was allowed to explore for 5 min. After the mouse had remained in the box for 5 min, it was removed, and 30 min later, the retention session began. One of the objects used in the training session was replaced with another object that was different from the former in shape and color, and the mouse was placed into the box again and allowed to explore for another 5 min. The floor of the box and the objects were cleaned with 75% ethanol every time before the mouse was placed in the box to prevent the build-up of olfactory cues. The time spent exploring the familiar and the novel objects was recorded by manual timing. It was regarded as a valid exploration when the nose of the mouse was touching the object or was directed toward the object at a distance of no more than 2 cm. Sitting on the object was not considered to be exploratory behavior (20). The relative discrimination index (DI) was calculated by subtracting the exploration time of the familiar object from that of the novel object divided by the total exploration time (21).
STPA test
We performed the STPA test as described by Liu et al. (22) with a slight modification. The apparatus was a box consisting of two chambers of equal size (20 cm × 12 cm × 60 cm). The chambers were divided into a light and a dark chamber, with the former equipped with an illuminator. Basically, the STPA was carried out in three sessions: the habituation trials, the acquisition trials and the retention sessions. In the habituation trials, the mouse was placed into the light chamber and allowed to shuttle freely between the two chambers. This session occurred 3 times (3 min per time), after which the mouse could only enter into the dark chamber within 15 s once placed into the light chamber. In the acquisition trials, the mouse was placed into the light chamber facing the door and could move freely. After habituating for 3 min in the apparatus, once the mouse stepped into the dark chamber with all its paws, a constant electric shock (constant voltage 50 V) was delivered to its feet. The computer software would record the time spent in the dark chamber, the number of times the mouse entered the dark chamber and the time spent near the connecting door over 5 min. The retention session was performed 24 h after the acquisition trials. The protocols were identical to those in the acquisition trials. The latency into the dark chamber, the time spent in the dark chamber and the time spent near the connecting door over 5 min were recorded by the software. If the mouse did not step into the other chamber within the 5 min, the latency was recorded as 300 s.
MWM test
The MWM test was carried out as described by Xu et al. (23) with a slight modification. This task consisted of three sessions: the hidden-platform acquisition, the probe trial and working memory testing (24). This test was conducted in a circular tank (100 cm diameter, 40 cm height) filled with water (22–23 °C) made opaque through the addition of nontoxic black ink. There was a platform invisible beneath the water’s surface that was 15 cm in height and 6 cm in diameter and that could be moved when necessary. The related swimming parameters were monitored by a video camera overhead and analyzed using an image analyzer and tracking system.
In the hidden-platform acquisition training, the platform was submerged 1 cm beneath the water’s surface. The mouse was placed into the water randomly from three different locations and underwent three successive trials per day. Before and after the training, the mouse was adapted on the platform for 15 s. If the mouse failed to find the location of the platform in 90 s, it was guided onto the platform and then allowed to remain there for 15 s. In the probe trial testing, the platform beneath the water was removed from the tank. The mouse was placed into the water from the quadrant opposite the previous platform and then was allowed to explore the previous platform freely over 90 s. The working memory testing lasted for 3 days, during which the platform was still invisible, but the position of the platform was changed every day. The mouse was tested 3 times per day and placed into the water from a different quadrant every time. Overall, the average latency of the second trial of each day was analyzed.
Western blotting analysis
A proper amount of protein extraction containing 1% protease inhibitors and 1% phosphatase inhibitors was added to the hippocampus sample that then underwent ultrasonic treatment 5–6 times until the sample was homogeneous. Next, the sample was centrifuged at 20,000 g for 20 min. The supernatant was collected and quantified by BCA protein assay. Twenty-five micrograms of quantified protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a nitrocellulose membrane. After 2 h of blocking in 7.5% milk, solution, the membrane was incubated with primary antibodies at 4 °C overnight, including anti-BDNF (1:2,000, Abcam), anti-TrkB (1:1,000, Abcam) and anti-ULK1 (1:1,000, Cell Signaling Technology) and anti-LC3B (1:1,000, Sigma-Aldrich). After washing three times (10 min each time), the membrane was incubated with appropriate horseradish peroxidase-linked secondary antibodies (1:5,000, ABclonal) at room temperature for another 2 h. Finally, the protein was detected by enhanced chemiluminescence. The gray intensity values of the bands were analyzed using ImageJ 1.46r software.
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). One-way ANOVA followed by post hoc LSD test was performed to determine the significance for multiple comparisons when the data met normal distribution. When the data did not meet the requirements of normal distribution, the Mann-Whitney U test was conducted. Significance was set at P<0.05. Calculations were performed using SPSS statistical software (version 17.0).
Results
Effects of estrogen deprivation on the memory of mice in the NOR test
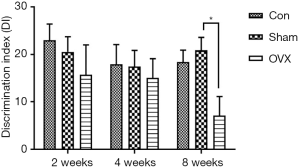
In the NOR test, a higher DI indicates a higher preference of the mice toward the novel object, reflecting better memory performance. As revealed by the DI in this test, no significant difference was observed between the Sham and Con groups, implying that the memory performance of OVX mice was not disrupted by the sham operation. With the prolongation of estrogen deprivation, the memory deficits in OVX mice were not observed until 8 weeks after the surgery. As shown in Figure 2, there was no remarkable difference between the Sham and OVX groups in DI at 2 and 4 weeks post-OVX. When the time was extended to 8 weeks, the DI in the OVX group (7.1%±4.0%) significantly decreased compared with that of the Sham group (20.8%±2.7%).
Effects of estrogen deprivation on the memory of mice in the STPA test
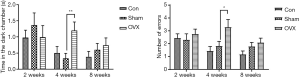
In the retention sessions of the STPA test, the time spent in the dark chamber and the number of errors made by the mice were analyzed at different time points after OVX. As shown in Figure 3, there was no significant difference between the Sham and OVX groups at 2 weeks post-OVX. However, at 4 weeks after OVX, remarkable increases in the time spent in the dark chamber and the number of errors were observed. Surprisingly, the performance of OVX animals did not deteriorate further with the increased duration of estrogen deprivation. When the duration of estrogen deprivation was extended to 8 weeks, no significant difference was found between the Sham and OVX groups in this test. We also monitored the performances of the mice in the acquisition trial test, and no significant difference was detected between the two groups in the time spent in the dark chamber or in the numbers of errors (data not shown).
Effects of estrogen deprivation on the memory of mice in the MWM test
The MWM test is a classical approach to studying the reference memory and working memory. Generally, the escape latencies in the hidden-platform acquisition training and the number of times the mice cross the platform or the time spent in the target quadrants in the probe trial testing can be used to evaluate the spatial reference memory of mice. In the hidden-platform acquisition training, estrogen deprivation did not lead to a prominent increase in escape latencies (Figure 4A). As shown in Figure 4B and C, no significant differences in crossing number or the time spent in the target quadrants were observed between the Sham and OVX groups in the probe trial testing either. Moreover, a slight decrease in crossing numbers was observed in the Con group at 4 weeks post-OVX, without significant differences compared to the Sham group, possibly due to the interference from metestrus or diestrus of the mice in the Con group. In summary, estrogen deprivation for up to 8 weeks did not affect the OVX mice’s reference memory.
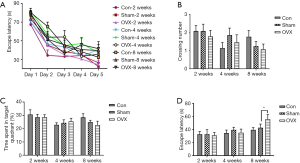
The hidden-platform acquisition training was also performed in our study. The escape latency in the working memory test (Figure 4D) did not show significant differences between the Sham and OVX groups at 2 or 4 weeks post-OVX. However, when the time was extended to 8 weeks post-OVX, the escape latency increased significantly from 36.1 to 59.9 s compared with the Sham group, which indicated working memory deficits in the OVX mice.
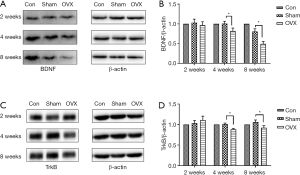
Effects of estrogen deprivation on the BDNF and TrkB levels in the hippocampus
The BDNF/TrkB signaling pathway is crucial to synaptic plasticity, which participates in memory formation by contributing to the changes in connectivity within neuronal circuits (7). We observed a significant decrease in BDNF (Figure 5A,B) and TrkB (Figure 5C,D) levels at 4 weeks post-OVX as demonstrated by Western blotting. When compared with the Sham group, the BDNF levels in the OVX animals decreased by 18.9% at 4 weeks post-OVX, while this gap increased to 39.0% when the duration of estrogen deprivation was extended to 8 weeks. Similarly, the TrkB protein levels decreased by 12.4% at 4 weeks post-OVX but this gap remained largely unchanged at 8 weeks post-OVX.
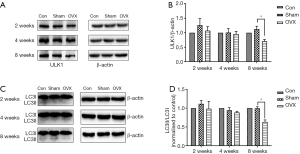
Effects of estrogen deprivation on ULK1 and LC3II/LC3I levels in the hippocampus
Previous studies have reported that BDNF could enhance autophagy. To determine whether autophagy was affected by estrogen deprivation, we detected the presence of ULK1 and LC3II/LC3I in the hippocampus. However, there was no significant change in this protein at 2 or 4 weeks post-OVX. At 8 weeks post-OVX, however, the ULK1 level decreased by 37.2% (Figure 6A,B), while the results of LC3II/LC3I decreased by 36.3% compared with the Sham group (Figure 6C,D).
Discussion
In this study, three different memory-related behavioral tests were used for the first time to investigate the effects of estrogen deprivation of different durations on memory changes. The results showed that the performance of the mice at each time point post-OVX differed between the behavioral tests. Memory impairment was observed as early as 4 weeks post-OVX in the STPA test, but when the estrogen deprivation was prolonged to 8 weeks, the mice showed a memory rebound in this test. However, in the NOR and MWM tests, which are both hippocampus-dependent tasks, memory impairments were detected in the mice at 8 weeks after OVX. These results inspired us to examine the influence of estrogen deprivation on different behavioral tests and their relevant mechanisms. In the current study, downregulation of the BDNF/TrkB signaling pathway in the hippocampus was observed at 4 and 8 weeks post-OVX, followed by a decrease in the autophagy-related protein ULK1 and LC3II/LC3I at 8 weeks post-OVX. We speculated that the downregulation of the BDNF/TrkB signaling pathway and the subsequent decrease in autophagy levels in the hippocampus might account for the progressive change in the hippocampus-dependent memory.
The poor performance of the mice in the NOR test reflects impairment of declarative memory (25). A considerable number of studies have demonstrated that memory impairment is induced by estrogen deprivation, while the duration of estrogen deprivation has varied widely between studies. One previous study found impaired memory in mice in the NOR test following one week of estrogen deprivation (26,27). However, another study by Fonseca et al. did not observe a significant difference in mouse memory between the Sham and OVX groups at 1 week post-OVX, while a significant decrease in DI was observed at 6 weeks post-OVX (28). A significant decrease in DI was also found at 4 weeks post-OVX (10). In our present study, however, it was not until 8 weeks post-OVX that the memory deficits were evident in the mice.
We also performed the MWM test, a classical method to evaluate the spatial memory of mice (29). A significant increase in latency to platform in the working memory test was observed at 8 weeks post-OVX. However, there was no significant difference between the Sham and OVX groups in the navigation test, which was used to evaluate the reference memory. Although there have been multiple studies reporting impaired reference memory in OVX animals (30-32), these results have been challenged by several findings. In a recent study, no significant differences between the Sham and OVX groups was observed in the MWM and Y-maze tests at 2 weeks post-OVX (5). Similarly, OVX did not influence the reference memory of mice at 5 weeks post-OVX when the mice were examined using the MWM test (33). Impaired working memory performance but unaffected spatial reference was also reported at 5 weeks post-OVX using the radial arm maze test (34). These study findings elicit a question: why does OVX impair the working memory but not influence the reference memory after a period of estrogen deprivation? This finding might be related to the reduction in acetylcholine (ACH) after OVX. As we know, ACH is a neurotransmitter produced by cholinergic cells. It is essential for processing learning and memory (35). Interestingly, one previous study reported that selectively killing cholinergic cells could impair the working memory (36), whereas it did not affect the spatial reference memory (37). Thus, further study is needed to investigate the changes in cholinergic cells after estrogen deprivation.
Surprisingly, a memory rebound was observed in the mice in the STPA test at 8 weeks after OVX. In fact, the effects of estrogen deficiency of different durations on memory remain controversial (38). In general, menopausal women have been considered to suffer from cognitive disorders as a result of estrogen decline. However, this viewpoint was challenged by a recent study that demonstrated that women aged 50–54.9 years had even poorer memory than older women (39). Their verbal memory did decline at the menopausal transition but probably rebounded during the postmenopausal stage (40). Researchers have tried to decipher these conflicting findings. Some scientists noted the negative relationship between verbal memory and vasomotor symptoms (VMS) and attributed the memory rebound to the disappearance of VMS (41). In addition, the local synthesis of estrogen in the brain might also contribute to the memory rebound. It has been reported that the local synthesized estrogen in the brain has a stronger effect on synaptic plasticity than peripheral estrogen (42). The locally synthesized estrogen in the brain might show a compensatory increase with the prolongation of estrogen deprivation, leading to the subsequent memory rebound in the mice. Except for the two reasons mentioned above, the regional distribution of estrogen in the brain might also play a crucial role. Although the estrogen deprivation led to a decrease in hippocampus activity, it might increase the activity of the anterior cingulate and dorsolateral prefrontal cortex (43). This compensatory mechanism outside the hippocampus in the brain might lead to the different outcomes in the STPA test and other behavioral tests. Overall, we suggest examining VMS, estradiol synthesized in the brain and the differential effects of estrogen on variable brain domains as future directions of study to clarify these discrepancies.
In our study, we found that there was no significant difference in the expression of BDNF and TrkB between the Sham and OVX groups at 2 weeks post-OVX. However, the mice in the OVX group showed a significant decrease in the expressions of the two proteins at 4 and 8 weeks after surgery. As we know, BDNF acts presynaptically via TrkB to regulate synaptic plasticity and influence the mnemonic effects of estrogen (44). Previous studies have reported that an increase in BDNF could restore spine actin polymerization and the stabilization of long-term potentiation in the hippocampus, which is crucial to spine plasticity (45). Additionally, blockade of the TrkB receptor was reported to abolish E2-mediated increases in excitability of the medial prefrontal cortex (mPFC) neurons (46). According to our results, the decrease in BDNF and TrkB levels implied that the beneficial effects of the BDNF/TrkB signaling pathway on the E2-induced enhancement of excitability and spine plasticity might be attenuated at 4 and 8 weeks post-OVX. However, the onset of this decrease in BDNF and TrkB levels preceded the beginning of hippocampus-dependent memory impairment. Our study also found a decrease in autophagy levels in OVX mice at 8 weeks after surgery. Notably, this decrease corresponded to the time point at which the OVX mice exhibited memory impairments in the NOR and MWM tests, but it was significantly after the BDNF levels decreased. We speculated that the preexisting alteration of BDNF levels at 4 weeks post-OVX might affect the autophagy process. In fact, studies have found a relationship between BDNF and autophagy. One previous study demonstrated that autophagy-associated proteins and BDNF were both upregulated following fluoxetine treatment in an unpredictable chronic mild stress mouse model of depression (47). With the treatment of BDNF in BMECs under hyperglycemia, the LC3II protein levels increased and the p62 levels decreased, indicating that the blockage of autophagy induced by hyperglycemia was alleviated by BDNF (48). Hence, we presumed that the downregulation of the BDNF/TrkB signaling pathway in our study led to autophagy suppression revealed by the decrease in ULK1 and LC3II/LC3I levels in the hippocampus, which further affected the memory function.
In conclusion, memory impairment may be induced in mice as early as 4 weeks post-OVX, but there is a possibility of memory rebound with the prolongation of estrogen deprivation, as 8 weeks of estrogen deprivation was more likely to induce hippocampus-dependent memory impairment. Since the BDNF level in the hippocampus decreased immediately after OVX, this protein might be a crucial biomarker for the further prediction of memory impairment induced by estrogen deficiency.
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China (No. 2018YFC1602105) and the National S&T Major Projects for New Drug Innovation and Development (No. 2017ZX09301029). The funding source had no further involvement in the process of study, writing of the report and the decision to submit the paper for publication.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Committee on Animal Care and Use of the Institution of Medical Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College (SLXD-20170718072).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moradi F, Jahanian Sadatmahalleh S, Ziaei S. The effect of hormone replacement therapy on cognitive function in postmenopausal women: An RCT. Int J Reprod Biomed (Yazd) 2019. [Crossref] [PubMed]
- Kiss A, Delattre AM, Pereira SI, et al. 17beta-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res 2012;227:100-8. [Crossref] [PubMed]
- Kalueff AV, Tuohimaa P. Experimental modeling of anxiety and depression. Acta Neurobiol Exp (Wars) 2004;64:439-48. [PubMed]
- Sarkaki A, Amani R, Badavi M, et al. Effect of ovariectomy on reference memory version of Morris water maze in young adult rats. Iran Biomed J 2008;12:123-8. [PubMed]
- Wilson IA, Puolivali J, Heikkinen T, et al. Estrogen and NMDA receptor antagonism: effects upon reference and working memory. Eur J Pharmacol 1999;381:93-9. [Crossref] [PubMed]
- Mander T. Long-term benefits and risks of HRT (Section 11): Dementia. Post Reprod Health 2016;22:95. [Crossref] [PubMed]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013;14:7-23. [Crossref] [PubMed]
- Lu B, Nagappan G, Guan X, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 2013;14:401-16. [Crossref] [PubMed]
- Liu Y, Fowler CD, Young LJ, et al. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol 2001;433:499-514. [Crossref] [PubMed]
- Aggarwal A, Sharma N, Sandhir R, et al. S-nitrosoglutathione prevents cognitive impairment through epigenetic reprogramming in ovariectomised mice. Biochem Pharmacol 2019;168:352-65. [Crossref] [PubMed]
- Levine B, Kroemer G. Autophagy in the Pathogenesis of Disease. Cell 2008;132:27-42. [Crossref] [PubMed]
- Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One 2010;5:e9979. [Crossref] [PubMed]
- Zhan L, Li J, Wei B. Autophagy in endometriosis: Friend or foe? Biochem Biophys Res Commun 2018;495:60-3. [Crossref] [PubMed]
- Fang YY, Zeng P, Qu N, et al. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J Neurochem 2018;146:703-21. [Crossref] [PubMed]
- Yao Q, Feng M, Yang B, et al. Effects of ovarian hormone loss on neuritic plaques and autophagic flux in the brains of adult female APP/PS1 double-transgenic mice. Acta Biochim Biophys Sin (Shanghai) 2018;50:447-55. [Crossref] [PubMed]
- Moreira SF, Nunes EA, Kuo J, et al. Hypoestrogenism alters mood: Ketamine reverses depressive-like behavior induced by ovariectomy in rats. Pharmacol Rep 2016;68:109-15. [Crossref] [PubMed]
- Fotsing D, Ngoupaye GT, Ouafo AC, et al. Effects of Gladiolus dalenii on the Stress-Induced Behavioral, Neurochemical, and Reproductive Changes in Rats. Front Pharmacol 2017;8:685. [Crossref] [PubMed]
- Nelson JF, Felicio LS, Randall PK, et al. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 1982;27:327-39. [Crossref] [PubMed]
- Takuma K, Hoshina Y, Arai S, et al. Ginkgo biloba extract EGb 761 attenuates hippocampal neuronal loss and cognitive dysfunction resulting from chronic restraint stress in ovariectomized rats. Neuroscience 2007;149:256-62. [Crossref] [PubMed]
- Uzbay T, Parlakpinar H, Akdag E, et al. Chronic melatonin treatment reverses disruption of prepulse inhibition in pinealectomized and pinealectomized-plus-ovariectomized rats. Behav Brain Res 2013;239:1-7. [Crossref] [PubMed]
- Shahidi S, Hashemi-Firouzi N, Asl SS, et al. Serotonin type 6 receptor antagonist attenuates the impairment of long-term potentiation and memory induced by Abeta. Behav Brain Res 2019;364:205-12. [Crossref] [PubMed]
- Liu YM, Li ZY, Hu H, et al. Tenuifolin, a secondary saponin from hydrolysates of polygalasaponins, counteracts the neurotoxicity induced by Abeta25-35 peptides in vitro and in vivo. Pharmacol Biochem Behav 2015;128:14-22. [Crossref] [PubMed]
- Xu P, Wang KZ, Lu C, et al. Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J Ethnopharmacol 2016;194:819-26. [Crossref] [PubMed]
- Vorhees CV, Makris SL. Assessment of learning, memory, and attention in developmental neurotoxicity regulatory studies: synthesis, commentary, and recommendations. Neurotoxicol Teratol 2015;52:109-15. [Crossref] [PubMed]
- Horiguchi M, Hannaway KE, Adelekun AE, et al. D(1) receptor agonists reverse the subchronic phencyclidine (PCP)-induced novel object recognition (NOR) deficit in female rats. Behav Brain Res 2013;238:36-43. [Crossref] [PubMed]
- Mitra S, Bastos CP, Bates K, et al. Ovarian Sex Hormones Modulate Compulsive, Affective and Cognitive Functions in A Non-Induced Mouse Model of Obsessive-Compulsive Disorder. Front Behav Neurosci 2016;10:215. [Crossref] [PubMed]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 2006;84:112-9. [Crossref] [PubMed]
- Fonseca CS, Gusmao ID, Raslan AC, et al. Object recognition memory and temporal lobe activation after delayed estrogen replacement therapy. Neurobiol Learn Mem 2013;101:19-25. [Crossref] [PubMed]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47-60. [Crossref] [PubMed]
- Rubio J, Qiong W, Liu X, et al. Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice. Evid Based Complement Alternat Med 2011;2011:253958. [Crossref] [PubMed]
- Uzum G, Bahcekapili N, Baltaci AK, et al. Chronic (3-Weeks) Treatment of Estrogen (17beta-Estradiol) Enhances Working and Reference Memory in Ovariectomized Rats: Role of Acetylcholine. Neurochem Res 2016;41:1468-74. [Crossref] [PubMed]
- Lee YB, Lee KH, Sohn HS, et al. Effects of soy phytoestrogens on reference memory and neuronal cholinergic enzymes in ovariectomized rats. J Med Food 2009;12:64-70. [Crossref] [PubMed]
- Singh M, Meyer EM, Millard WJ, et al. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 1994;644:305-12. [Crossref] [PubMed]
- Cao F, Zhang H, Meng X, et al. Ovariectomy-mediated impairment of spatial working memory, but not reference memory, is attenuated by the knockout of the dopamine D 3 receptor in female mice. Behav Brain Res 2013;247:27-33. [Crossref] [PubMed]
- Francis PT. The interplay of neurotransmitters in Alzheimer's disease. CNS Spectr 2005;10:6-9. [Crossref] [PubMed]
- Walsh TJ, Herzog CD, Gandhi C, et al. Injection of IgG 192-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res 1996;726:69-79. [Crossref] [PubMed]
- Shen J, Barnes CA, Wenk GL, et al. Differential effects of selective immunotoxic lesions of medial septal cholinergic cells on spatial working and reference memory. Behav Neurosci 1996;110:1181-6. [Crossref] [PubMed]
- Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser 1996;866:1-107. [PubMed]
- Webster AD, Finstad DA, Kurzer MS, et al. Quality of life among postmenopausal women enrolled In the Minnesota Green Tea Trial. Maturitas 2018;108:1-6. [Crossref] [PubMed]
- Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab 2013;98:3829-38. [Crossref] [PubMed]
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause 2008;15:848-56. [Crossref] [PubMed]
- Kretz O, Fester L, Wehrenberg U, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci 2004;24:5913-21. [Crossref] [PubMed]
- Bayer J, Rune G, Schultz H, et al. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology 2015;56:213-25. [Crossref] [PubMed]
- Contreras-Zárate MJ, Day NL, Ormond DR, et al. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene 2019;38:4685-99. [Crossref] [PubMed]
- Kramár EA, Chen LY, Lauterborn JC, et al. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging 2012;33:708-19. [Crossref] [PubMed]
- Yousuf H, Smies CW, Hafenbreidel M, et al. Infralimbic Estradiol Enhances Neuron Excitability and Facilitates Extinction of Cocaine Seeking in Female Rats via a BDNF/TrkB Mechanism. Front Behav Neurosci 2019;13:168. [Crossref] [PubMed]
- Tan X, Du X, Jiang Y, et al. Inhibition of Autophagy in Microglia Alters Depressive-Like Behavior via BDNF Pathway in Postpartum Depression. Front Psychiatry 2018;9:434. [Crossref] [PubMed]
- Jin H, Zhu Y, Li Y, et al. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis 2019;24:511-28. [Crossref] [PubMed]


