
Induced pluripotent stem cells for the treatment of liver diseases: challenges and perspectives from a clinical viewpoint
Introduction
Despite the fact that current medical and surgical therapies are available for early stages of liver diseases, a significant clinical need exists for alternative treatments of intractable liver diseases. Cell-based therapies are being developed as promising tools, alternative to liver transplantation (LT), to treat degenerative disorders, inborn hepatic metabolic diseases and organ failure (1-5). The earliest attempts made in this field involved the transplantation of allogeneic hepatocytes which is hindered by the increasing shortage of suitable donor livers for hepatocyte isolation as well as by the insufficient functional quality and great susceptibility to cryopreservation and thawing of hepatocytes (2,3,6). Therefore, the major challenge for hepatic cell therapy is to identify alternative reliable cell sources, equivalent to hepatocytes, expandable, bankable and engraftable, which can be derived from reproducible methods, thus making them available for transplantation to large numbers of patients. Current research on induced pluripotent stem cells (iPSCs), which are adult cells that have been genetically reprogrammed to an embryonic stem cell (ESC)-like state, point to these cells as an appealing option to face these challenges. iPSCs possess the unique properties of hepatic differentiation, self-renewal and in vitro expansion which make them a very promising cell source for generating large-scale production of suitable functional hepatocyte-like cells (HLCs) (1,4,7,8). Large numbers of HLCs can be made readily available to any patient on an as-needed basis for hepatic cell-based therapies, both for programmed treatment for liver-based metabolic disorders and emergency use in patients with acute liver failure (ALF), acute-on-chronic liver failure (ACLF) or end-stage liver disease (ESLD) (4,7-10).
On the other hand, using patient-specific iPSCs has been made available likely solving the problem of allogeneic immune rejection. In this case, ex vivo gene-corrected patient-specific iPSCs lines raise the possibility of autologous transplantation for the treatment of hereditary liver metabolic diseases (10,11). Ideally, these cell lines should be highly viable preparations with robust hepatic function and engraftment capacity. Recent preclinical studies have shown that transplantation with HLCs differentiated from human iPSCs ameliorated inherited liver diseases (12,13) and ALF (14). Nonetheless, critical aspects to be addressed in clinical trials are long-term safety, tolerability, efficacy as well as the tumorigenic potential of the iPSC-derived cell based treatments to define the target patient and standardize the protocols.
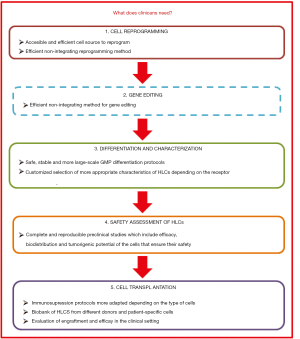
This review focuses on the different strategies recently described to reprogram somatic cells to the pluripotency, their differentiation to HLCs and their potential use to provide a real prospect of bringing cell-based therapy for liver diseases in two main areas: to make unlimited numbers of HLCs available to extend treatments to many patients and to treat hereditary liver diseases using autologous genetically corrected HLCs (Figure 1). The review also reflects about challenges and uncertainties of their clinical application and the needs of clinicians (Figure 2).
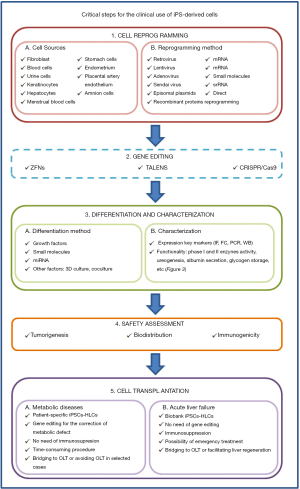
iPSC derivation
Based on the findings showing that Oct3/4, Sox-2 and Nanog play essential roles in the maintenance of early embryos and embryonic stem cells (ESCs) (15-17), Yamanaka’s group selected a pool of 24 genes to identify the reprogramming factors that could induce pluripotency in somatic cells. Finally, they selected the now known as Yamanaka factors (Oct3/4, Sox2, c-Myc and Klf-4) as they were able to successfully reprogram mouse fibroblasts into iPSCs (18). A year later they reprogrammed human fibroblasts into iPSCs by using the same combination of factors (19).
Since Yamanaka reported the first generation of iPSCs from somatic cells, two major aspects have been continuously under investigation: methods to induce somatic cell reprogramming and which somatic cells to reprogram. Currently, iPSCs can be obtained from different cell sources and through distinct strategies that have been reviewed in detail (8).
Cell sources for iPSCs derivation
Although initially iPSCs were obtained from fibroblasts, distinct cell sources have been used to derive them [for a review (20,21)]. Skin fibroblasts are simple to culture and easily accessible with a skin biopsy, although, since it is an invasive process, alternative cell sources have been explored. These alternative cells such as blood cells, urine cells, hair-follicle derived keratinocytes, menstrual blood cells or amnion cells have been derived into iPSCs.
Reprogramming methods
Important efforts have been made to understand the reprogramming process involved in the generation of iPSCs [reviewed by (20)]. The first method, used for the introduction of reprogramming transcription factors to human differentiated cells, was based on retroviral vectors (19), although, upon transduction, retroviral vectors are randomly integrated into the host genome, increasing the risk of tumor formation, which would be unsuitable for the generation of clinical-grade iPSCs.
For the future clinical applicability of iPSCs-derived cells, the development of non-integrative iPSCs derivation methods that do not introduce genetic changes is required. Among these the use of adenoviral vectors (22) or TransGen-free induction of human pluripotent stem cells (PSCs) by the vectors derived from Sendai virus (23) have been explored. The use of Sendai virus involves viral particles raising questions about the safety of the generated iPSCs. Due to this, others have focused their research on DNA-free and viral-free protocols based on the introduction of reprogramming-inducing molecules into cells such as recombinant proteins (24), microRNA (25), mRNA (26) or small molecule-mediated reprogramming (27). It has recently compared the efficiency of different RNA-based footprint methods through the use of self-replicating RNA (srRNA) and the use of synthetic mRNA, showing that srRNA had a better efficiency indicating that could be an appropriate approach for clinical applications (28). It should be also considered that direct reprogramming of somatic cells into HLCs using lentiviral vectors (29,30) has been proposed.
On the other hand, recently, human primary hepatocytes have been reprogrammed into a population of proliferating bipotent cells with regenerative potential by adding two small molecules and HGF, providing a new tool for personalised cell-based therapy (31). Finally, it should be considered that the iPSCs derivation efficiency of all the proposed methods is low. Improving both the reprogramming efficiency and safety using integration-free and virus-free methods under feeder-free conditions is the most promising step in the safe translation of iPSCs to their clinical application in personalized regenerative medicine.
iPSCs correction
The gene correction of patient-specific iPSCs should be applied for patients with monogenic inherited metabolic diseases such as Crigler-Najjar disease or alpha-1 antitrypsin (A1AT) deficiency. The most widely used tools for genome editing are zinc-finger endonucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated system (Cas9) [reviewed by (32)]. After gene editing, iPSCs could be differentiated into HLCs and then transplanted. In this sense, it has been described the gene correction of A1AT deficiency in iPSCs by combining two technologies: ZFNs and PiggyBac technology which resulted in the in vitro restoration of the structure and function (33).
In another study, researchers generated iPSCs from a patient with Wilson’s disease (with a mutation in the ATPase Cu2+ transporting beta polypeptide gene) and corrected them using a lentiviral vector. These corrected iPSCs differentiated into HLCs showed copper metabolism capacity (34). In a study of Omer et al. [2017] the CRISPR/Cas9 system was used to correct a LDLR mutation of iPSCs derived from a patient with hypercholesterolemia. In this case, the genetic correction restored LDLR-mediated endocytosis (35).
The efficacy of gene editing has shown the potential for the application of patient-derived iPSCs for the correction of underlying genetic defects that could allow the autologus transplantation and, thus, reduce problems of immune rejection.
iPSC differentiation, characterization and safety
PSCs, including both ESCs and iPSCs can differentiate into all cell types of the body and are a promising tool for regenerative medicine, drug discovery and development studies. Great advances have been made to differentiate initially ESCs and now also iPSCs toward the hepatocyte lineage although the maturation levels and the characterization of HLCs vary in different studies.
Differentiation of iPSCs into HLCs
The generation of HLCs from iPSCs is a complex process. Although several protocols have been defined for the generation of HLCs from PSCs, this process includes 3 basic stages by administering different soluble factors (i.e., growth factors) in a time-dependent manner to mimic ontogenetic liver development (9,36). The first step includes endoderm induction by Activin A, BMP4, LY294002 and Wnt3a. The second step uses as inducers FGF2, FGF4 and BMP4 to produce hepatoblast cells (hepatic specification), whereas the final step is hepatic differentiation and maturation (using HGF and oncostatin M). For clinical applications HLCs need to be produced in a large scale and different bioreactors have been proposed (1,37).
In addition to the use of growth factors, some groups have proposed the use of microRNAs (38) or small molecules (39) to differentiate PSCs into HLCs as a simple, highly efficient, and cost-effective alternative for generation. On the other hand, different improvements of the standard protocols such as co-culture with other cell types (40), genetic manipulation (41) and/or culture in 3D configuration (42) trying to simulate what happens in vivo have been also proposed. Although even a 2-fold increase in some of the functions has been demonstrated with some of these new methods (42), HLCs-derived iPSCs are closer to a fetal than adult hepatocyte phenotype and in other cases there is a lack of appropriate controls that allow a clear conclusion about the results. Moreover, it has been described that the origin of the donor cells and not the derivation method can determine the variation in hepatic differentiation (43), which really complicates the therapeutic use of iPSCs-HLCs because the quality of HLC could be different depending on the donor.
Characterization of HLCs
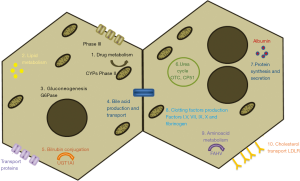
Hepatocytes are the most predominant cell type of the adult liver mass and perform essential functions, including plasma protein secretion, ureogenesis, metabolic homeostasis or detoxification (37). Figure 3 summarizes important hepatic specific functions that HLCs should exhibit although, depending on the disease to be treated with these cells, the studies may focus on the lack of a specific liver function. For example, in the case of the treatment of patients receiving extensive medication, clinicians may focus on CYPs activities and drug-metabolism enzymes, whereas for the treatment of inborn metabolic errors, characterization should focus on the specific lacking function. Although numerous studies have demonstrated that iPSCs can be differentiated into HLCs, the characterization of the cells sometimes is only based on the analysis of the expression of few hepatic markers by means of immunofluorescence or RT-PCR and do not include functional analysis. Before their clinical use, specific functional assays should be also routinely included and standardized. Moreover, it should be also considered that cells should be phenotypically stable over a long period and safe before being applied clinically (1) and that in vivo maturation is expected (44), which may compensate the lack of a fully mature phenotype.
Safety assessment of HLCs
Complete characterization of the inherent immunogenicity profiles of iPSCs is also essential to define the best immunosuppressive strategy to favour their homing and engraftment (45). On the other hand, genetic modifications have profound functional implications and promote tumorigenic qualities, such as increased proliferation or higher frequencies of tumor-initiating cells (10). In this sense, the development of well-defined methods to reduce the expression of oncogenic genes in iPSCs is necessary to reduce the tumorigenicity of transplanted cells (46). The prospective removal (e.g., removal before transplantation) of tumorigenic cells using surface antigens has been proposed and would provide the highest level of safety (47). Finally, the safe distribution of the cells should be also assessed and it has been proved in different animal models.
Challenges of HLCs derived from iPSCs
Long-term safety, tolerability and efficacy of iPSCs-derived hepatic cell-based treatments are key issues to be addressed prior to the translation of cell therapy to the clinical practice. Human iPSCs technology is still in its infancy and a number of hurdles need to be overcome before these cell therapies become a reality. Reprogramming itself can induce both genetic and epigenetic defects in iPSCs (48), and it is possible that those defects can directly or indirectly promote immunogenicity and tumorigenicity in vivo, raising safety concerns. In fact, it has been recently reported that the genomic translocation detected in the iPSCs will create fusion proteins and new immunogenic determinants (49).
Immunogenicity of HLCs derived from iPSCs
Theoretically, the autologous HLCs derived from a patient should be immune tolerant without any concern of immune rejections after transplantation into the same patient (50). However, it has been reported that even syngeneic iPSC-derived cells can be immunogenic in syngeneic hosts by using a teratoma transplantation model (51).
On the other hand, it has been shown a differential immune recognition between differentiated and undifferentiated pluripotent cells (11,46,52). Undifferentiated, but not differentiated, PSCs have been reported to possess immune privilege properties, and would thus be less susceptible to immune recognition than their derived differentiated cell types (46,52). iPSCs are epigenetically abnormal and inherited epigenetic signature of parental cells could explain abnormal expression of immunogenic proteins expressed during the differentiation of iPSCs (53). In fact, undifferentiated iPSCs show a lower expression of MHC class I, and the complete absence of MHC class II antigens compared to their differentiated progeny (54). Generation of normal HLA-typed iPSCs banks homozygous for HLA-A, -B, and -DR, the most important loci to match, could be a solution to use compatible donors and reduce allograft rejection (55). Nevertheless, further in vivo studies will be needed to determine to what extent appropriately and terminally differentiated pluripotent cell lineages will induce the immunoresponse after transplantation (10).
Tumorigenicity and safety of gene editing
Genome editing has evolved to address the need for improving the efficiency and specificity of traditional genome modification achieved by homologous recombination. However, due to the possibility of off-target effects (edits in the wrong place) and mosaicism (when some cells carry the edit but others do not), safety is of primary concern. An important safety issue for genome editing is the accurate assessment of off-target cleavage by endonucleases and the effects of non-specific activity (56). The enhancement to efficiency and safety of genome editing will bring cell-based therapies closer to the clinic for patients with inborn metabolic diseases (56).
In this context, tumorigenicity is a serious bottleneck for developing individualized hepatic cell therapy using patients’ own or compatible banked iPSCs (48,49). The intrinsic qualities of self-renewal and pluripotency that make PSCs so therapeutically promising are also responsible for an equally fundamental tumorigenic potential. The induction of pluripotency by reprogramming somatic cells has been linked to tumorigenic transformation by creating genomic aberrations at chromosomal and sub-chromosomal stochastically generated levels (57). This genomic instability of iPSCs can create new immunogenic determinants like the tumor antigens developed in cancer cells (48,49). The potential risk of tumorigenicity has been evaluated in recent years in small and large animal studies. Ultimately, genetic modifications can promote tumorigenic qualities, such as increased proliferation, growth factor independence and higher frequency of tumor-initiating cells.
Protocols for the elimination of the remaining PSCs in the HLCs cultures have been described but the risk of teratoma formation after transplantation remains and could be an obstacle for clinical grade manufacturing (58).
Engraftment of transplanted cells
Engraftment potential of iPSC-HLCs, both short- and long-term, is another relevant key issue associated with the success of hepatic cell therapy. The proliferative advantage of transplanted native hepatocytes over resident hepatocytes to efficiently repopulate the liver has been shown in a number of animal models (59). Concerns associated with safety and engraftment potential of iPSC-HLCs are currently being addressed using these animal models. Among the many strategies aimed at increasing homing, engraftment and proliferation of transplanted cells, partial reversible embolization of the portal vein (60) and irradiation of the native liver could be applicable to human iPSC-HLCs therapy (61).
Large-scale and GMP production of HLCs
Although stem cell technology offers multiple treatment options, important technical advances are necessary before the clinical application of HLCs derived from iPSCs. In this sense, the starting material should be obtained and processed under good manufacturing practices (GMP) guidelines (62). Additionally, maintenance, expansion and differentiation of iPSCs will require GMP compatible conditions. Generation of HLA-typed iPSCs banks will lead to minimize the risk of allograft rejection. Finally, challenges remain to generate large quantity of well-differentiated cells to achieve enough material for transplantation and the obvious tremendous cost of getting enough tissue mass to maintain the hepatic functionality (63) On the other hand, a total of 2.0×108 viable cells/kg for each patient has been proposed as an optimal and safe dose in humans (64), but, considering that most of the patients are children and a possible future routine use, the costs would be reduced. Alternatively, it has been proposed the use of rats as bioreactors to get the sufficient amount of cells (65).
Potential therapeutic use of iPSCs in liver diseases
The demand for LT outweighs supply which leads to an increased morbidity and mortality among waiting-list patients. Cell-based liver therapies are envisaged as a useful therapeutic option to replace or complement whole organ transplantation by recovering and stabilizing the lost metabolic function for acute and chronic liver diseases (Table 1). However, success is hampered by the scarce availability of liver tissue to isolate good-quality cells, the low engraftment capability of the cells into the host liver mainly due to the rejection of transplanted cells as well as the difficulties to monitor and predict rejection (64). Human iPSCs differentiated towards the hepatic lineage could establish the basis for producing autologous cell therapies that would avoid immune rejection but that would require gene correction and/or help to create biobanks of readily available HLCs for the emergency treatment of ALF. Table 1 summarizes liver diseases susceptible to being treated with hepatic cell transplantation.
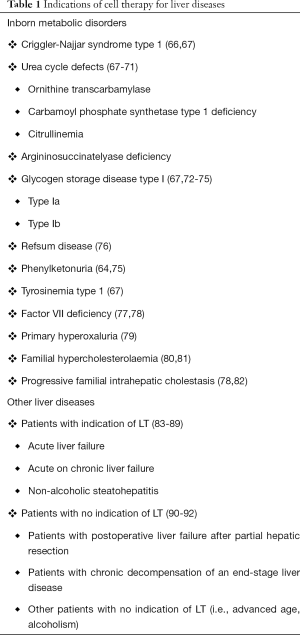
Full table
Inborn metabolic diseases
Liver-based inborn metabolic disorders are rare diseases characterized by defects in hepatic enzymes or proteins with metabolic functions, such as receptors or transporters. The management of patients with metabolic diseases is complex and LT may not always be the first therapeutic option in children due to invasiveness, recipient’s age or the need of lifelong immunosuppressive therapy (93,94). For those patients for whom the risks of LT are not justified, cell transplantation could be an appropriate therapeutic option to provide the missing liver function without replacing the whole organ. In this sense, hepatocyte transplantation has been used in pediatric patients with a number of inborn hepatic metabolic disorders (Crigler-Najjar disease, deficiencies in enzymes of the urea cycle, AAT1 deficiency) (93,94) with encouraging results.
Cell therapy for hereditary liver diseases with patient-specific iPSC-derived HLCs would require gene correction before or after reprogramming. Patient-specific iPSCs are considered a promising alternative for an ex vivo gene therapy approach that could be used for cell therapy applications and curing diseases. Personalized cell therapy using iPSCs would likely avoid rejection, and thus immunosuppression, which would be an important advantage over LT and hepatocyte transplantation. However, the immunogenicity of iPSCs and their derivatives is still controversial (50,51).
Other liver diseases
Hepatocyte transplantation has also been foreseen as a useful therapeutic approach for bridging patients to LT and is indicated for providing metabolic support during ALF and ACLF in which the only hope for survival for most patients is either LT, or facilitating liver regeneration of the native organ (93) (Table 1). Additionally, some patients suffering from ESLD, but with preserved liver function and no indication of LT could benefit from HLCs transplantation because cell therapy could delay disease progression and associated complications (95). Recently, the use of this strategy has been proposed to reverse the inflammation and fibrosis in non-alcoholic fatty liver disease, one of the commonest chronic liver diseases (96).
In these cases, time to make, mature and expand patient’s cells into iPSCs and then into HLCs may be prohibitive to particularly ALF treatment (8). For this reason, an allogeneic source of HLCs should be prepared for and readily available for their use in the treatment of ALF or ACLF which would imply the use of immunosuppression as when human hepatocytes are transplanted.
Final remarks
There is an urgent requirement for an unlimited source of human hepatocytes for transplantation that could be solved by using HLCs derived from iPSCs. The current challenge in this field is to develop reliable processes to differentiate stem cells into functional and engraftable cells that exhibit phenotypic stability and with no risk of tumorigenicity. Key issues should be addressed to improve clinical outcomes of hepatic cell therapy: (I) development of well-defined methods to generate iPSCs without viral integration and reduce the expression of oncogenic genes, (II) evaluation of unpredictable risks of using gene editing, (III) refinement of protocols for their complete and safe hepatic differentiation into HLCs comparable to their in vivo counterparts, (IV) expansion and production of cells under GMP conditions, (V) creation of iPSCs and HLCs biobanks, (VI) prospective removal (e.g., removal before transplantation) of tumorigenic cells through utilizing intrinsic cell properties, such as surface antigens, to minimize the tumorigenicity of transplanted cells, (VII) optimization and refinement of immunological strategies for transplant recipients, (VIII) defining preconditioning treatments of the recipient liver to enhance the engraftment and proliferation of donor cells, and (IX) development of non-invasive and accurate tracking or monitoring methods for cell survival and engraftment post-transplantation. Finally, such new strategies should be rigorously tested and validated in preclinical studies before they can be safely transferred to clinical practice with patients.
Acknowledgments
The authors acknowledge financial support from the ALIVE Foundation.
Funding: This work has been supported by the Institute of Health Carlos III (Plan Estatal de I+D+i 2013-2016) and cofinanced by the European Regional Development Fund “A way to achieve Europe” (FEDER) through grant CP16/00097 and PI18/00993. L Tolosa was supported by ISCIII CP16/00097.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.164). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol 2015;62:S157-69. [Crossref] [PubMed]
- Hansel MC, Davila JC, Vosough M, et al. The Use of Induced Pluripotent Stem Cells for the Study and Treatment of Liver Diseases. Curr Protoc Toxicol 2016;67:14.13.1-27.
- Iansante V, Mitry RR, Filippi C, et al. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res 2018;83:232-40. [Crossref] [PubMed]
- Nicolas CT, Hickey RD, Chen HS, et al. Concise Review: Liver Regenerative Medicine: from Hepatocyte Transplantation to Bioartificial Livers and Bioengineered Grafts. Stem Cells 2017;35:42-50. [Crossref] [PubMed]
- Tao YC, Wang ML, Chen EQ, et al. Stem Cells Transplantation in the Treatment of Patients with Liver Failure. Curr Stem Cell Res Ther 2018;13:193-201. [Crossref] [PubMed]
- Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant 2004;4 Suppl 6:7-13. [Crossref] [PubMed]
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol 2015;3:2. [Crossref] [PubMed]
- Yu Y, Wang X, Nyberg SL. Application of Induced Pluripotent Stem Cells in Liver Diseases. Cell Med 2014;7:1-13. [Crossref] [PubMed]
- Hannan NR, Segeritz CP, Touboul T, et al. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc 2013;8:430-7. [Crossref] [PubMed]
- Tolosa L, Pareja E, Gomez-Lechon MJ. Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases? Transplantation 2016;100:2548-57. [Crossref] [PubMed]
- Guha P, Morgan JW, Mostoslavsky G, et al. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 2013;12:407-12. [Crossref] [PubMed]
- Chen Y, Li Y, Wang X, et al. Amelioration of Hyperbilirubinemia in Gunn Rats after Transplantation of Human Induced Pluripotent Stem Cell-Derived Hepatocytes. Stem Cell Reports 2015;5:22-30. [Crossref] [PubMed]
- Yang J, Wong LY, Tian XY, et al. A Familial Hypercholesterolemia Human Liver Chimeric Mouse Model Using Induced Pluripotent Stem Cell-derived Hepatocytes. J Vis Exp 2018. [Crossref] [PubMed]
- Zhu S, Rezvani M, Harbell J, et al. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 2014;508:93-7. [Crossref] [PubMed]
- Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003;17:126-40. [Crossref] [PubMed]
- Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003;113:631-42. [Crossref] [PubMed]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000;24:372-6. [Crossref] [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Chhabra A. Derivation of Human Induced Pluripotent Stem Cell (iPSC) Lines and Mechanism of Pluripotency: Historical Perspective and Recent Advances. Stem Cell Rev 2017;13:757-73. [Crossref] [PubMed]
- Gerbal-Chaloin S, Funakoshi N, Caillaud A, et al. Human induced pluripotent stem cells in hepatology: beyond the proof of concept. Am J Pathol 2014;184:332-47. [Crossref] [PubMed]
- Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science 2008;322:945-9. [Crossref] [PubMed]
- Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 2009;85:348-62. [Crossref] [PubMed]
- Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009;4:381-4. [Crossref] [PubMed]
- Miyoshi N, Ishii H, Nagano H, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 2011;8:633-8. [Crossref] [PubMed]
- Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010;7:618-30. [Crossref] [PubMed]
- Shi Y, Desponts C, Do JT, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 2008;3:568-74. [Crossref] [PubMed]
- Steinle H, Weber M, Behring A, et al. Generation of iPSCs by Nonintegrative RNA-Based Reprogramming Techniques: Benefits of Self-Replicating RNA versus Synthetic mRNA. Stem Cells Int 2019;2019:7641767.
- Grath A, Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J Biol Eng 2019;13:14. [Crossref] [PubMed]
- Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 2014;14:370-84. [Crossref] [PubMed]
- Kim Y, Kang K, Lee SB, et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol 2019;70:97-107. [Crossref] [PubMed]
- Chang CY, Ting HC, Su HL, et al. Combining Induced Pluripotent Stem Cells and Genome Editing Technologies for Clinical Applications. Cell Transplant 2018;27:379-92. [Crossref] [PubMed]
- Yusa K, Rashid ST, Strick-Marchand H, et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature 2011;478:391-4. [Crossref] [PubMed]
- Zhang S, Chen S, Li W, et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson's disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet 2011;20:3176-87. [Crossref] [PubMed]
- Omer L, Hudson EA, Zheng S, et al. CRISPR Correction of a Homozygous Low-Density Lipoprotein Receptor Mutation in Familial Hypercholesterolemia Induced Pluripotent Stem Cells. Hepatol Commun 2017;1:886-98. [Crossref] [PubMed]
- Tolosa L, Caron J, Hannoun Z, et al. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res Ther 2015;6:246. [Crossref] [PubMed]
- Chen C, Soto-Gutierrez A, Baptista PM, et al. Biotechnology Challenges to In Vitro Maturation of Hepatic Stem Cells. Gastroenterology 2018;154:1258-72. [Crossref] [PubMed]
- Deng XG, Qiu RL, Wu YH, et al. Overexpression of miR-122 promotes the hepatic differentiation and maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive feedback loop. Liver Int 2014;34:281-95. [Crossref] [PubMed]
- Asumda FZ, Hatzistergos KE, Dykxhoorn DM, et al. Differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules. Differentiation 2018;101:16-24. [Crossref] [PubMed]
- Takebe T, Zhang RR, Koike H, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc 2014;9:396-409. [Crossref] [PubMed]
- Takayama K, Morisaki Y, Kuno S, et al. Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc Natl Acad Sci U S A 2014;111:16772-7. [Crossref] [PubMed]
- Gieseck RL 3rd, Hannan NR, Bort R, et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One 2014;9:e86372. [Crossref] [PubMed]
- Kajiwara M, Aoi T, Okita K, et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci U S A 2012;109:12538-43. [Crossref] [PubMed]
- Fourrier A, Delbos F, Menoret S, et al. Regenerative cell therapy for the treatment of hyperbilirubinemic Gunn rats with fresh and frozen human induced pluripotent stem cells-derived hepatic stem cells. Xenotransplantation 2020;27:e12544. [Crossref] [PubMed]
- Chhabra A. Inherent Immunogenicity or Lack Thereof of Pluripotent Stem Cells: Implications for Cell Replacement Therapy. Front Immunol 2017;8:993. [Crossref] [PubMed]
- Tan Y, Ooi S, Wang L. Immunogenicity and tumorigenicity of pluripotent stem cells and their derivatives: genetic and epigenetic perspectives. Curr Stem Cell Res Ther 2014;9:63-72. [Crossref] [PubMed]
- Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 2013;19:998-1004. [Crossref] [PubMed]
- Liu X, Li W, Fu X, et al. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front Immunol 2017;8:645. [Crossref] [PubMed]
- Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev 2017;13:7-16. [Crossref] [PubMed]
- Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013;494:100-4. [Crossref] [PubMed]
- Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212-5. [Crossref] [PubMed]
- Saljo K, Barone A, Molne J, et al. HLA and Histo-Blood Group Antigen Expression in Human Pluripotent Stem Cells and their Derivatives. Sci Rep 2017;7:13072. [Crossref] [PubMed]
- Zhao T, Zhang ZN, Westenskow PD, et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell 2015;17:353-9. [Crossref] [PubMed]
- Chen HF, Yu CY, Chen MJ, et al. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and their derivatives. Cell Transplant 2015;24:845-64. [Crossref] [PubMed]
- Taylor CJ, Peacock S, Chaudhry AN, et al. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 2012;11:147-52. [Crossref] [PubMed]
- Haeussler M, Schonig K, Eckert H, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol 2016;17:148. [Crossref] [PubMed]
- Laurent LC, Ulitsky I, Slavin I, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 2011;8:106-18. [Crossref] [PubMed]
- Cantz T, Sharma AD, Ott M. Concise review: cell therapies for hereditary metabolic liver diseases-concepts, clinical results, and future developments. Stem Cells 2015;33:1055-62. [Crossref] [PubMed]
- Basma H, Soto-Gutierrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 2009;136:990-9. [Crossref] [PubMed]
- Dagher I, Nguyen TH, Groyer-Picard MT, et al. Efficient hepatocyte engraftment and long-term transgene expression after reversible portal embolization in nonhuman primates. Hepatology 2009;49:950-9. [Crossref] [PubMed]
- Yamanouchi K, Zhou H, Roy-Chowdhury N, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology 2009;49:258-67. [Crossref] [PubMed]
- Asgari S, Pournasr B, Salekdeh GH, et al. Induced pluripotent stem cells: a new era for hepatology. J Hepatol 2010;53:738-51. [Crossref] [PubMed]
- Habka D, Mann D, Landes R, et al. Future Economics of Liver Transplantation: A 20-Year Cost Modeling Forecast and the Prospect of Bioengineering Autologous Liver Grafts. PLoS One 2015;10:e0131764. [Crossref] [PubMed]
- Soltys KA, Setoyama K, Tafaleng EN, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol 2017;66:987-1000. [Crossref] [PubMed]
- Agarwal N, Popovic B, Martucci NJ, et al. Biofabrication of Autologous Human Hepatocytes for Transplantation: How Do We Get There? Gene Expr 2019;19:89-95. [Crossref] [PubMed]
- Ambrosino G, Varotto S, Strom SC, et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant 2005;14:151-7. [Crossref] [PubMed]
- Ribes-Koninckx C, Ibars EP, Calzado Agrasot MA, et al. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant 2012;21:2267-82. [Crossref] [PubMed]
- Legido-Quigley C, Cloarec O, Parker DA, et al. First example of hepatocyte transplantation to alleviate ornithine transcarbamylase deficiency, monitored by NMR-based metabonomics. Bioanalysis 2009;1:1527-35. [Crossref] [PubMed]
- Meyburg J, Das AM, Hoerster F, et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation 2009;87:636-41. [Crossref] [PubMed]
- Stephenne X, Najimi M, Smets F, et al. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant 2005;5:2058-61. [Crossref] [PubMed]
- Stephenne X, Najimi M, Sibille C, et al. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology 2006;130:1317-23. [Crossref] [PubMed]
- Defresne F, Tondreau T, Stephenne X, et al. Biodistribution of adult derived human liver stem cells following intraportal infusion in a 17-year-old patient with glycogenosis type 1A. Nucl Med Biol 2014;41:371-5. [Crossref] [PubMed]
- Lee KW, Lee JH, Shin SW, et al. Hepatocyte transplantation for glycogen storage disease type Ib. Cell Transplant 2007;16:629-37. [Crossref] [PubMed]
- Muraca M, Gerunda G, Neri D, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet 2002;359:317-8. [Crossref] [PubMed]
- Stephenne X, Debray FG, Smets F, et al. Hepatocyte transplantation using the domino concept in a child with tetrabiopterin nonresponsive phenylketonuria. Cell Transplant 2012;21:2765-70. [Crossref] [PubMed]
- Sokal EM, Smets F, Bourgois A, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation 2003;76:735-8. [Crossref] [PubMed]
- Dhawan A, Mitry RR, Hughes RD, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation 2004;78:1812-4. [Crossref] [PubMed]
- Quaglia A, Lehec SC, Hughes RD, et al. Liver after hepatocyte transplantation for liver-based metabolic disorders in children. Cell Transplant 2008;17:1403-14. [Crossref] [PubMed]
- Guha C, Yamanouchi K, Jiang J, et al. Feasibility of hepatocyte transplantation-based therapies for primary hyperoxalurias. Am J Nephrol 2005;25:161-70. [Crossref] [PubMed]
- Barahman M, Zhang W, Harris HY, et al. Radiation-primed hepatocyte transplantation in murine monogeneic dyslipidemia normalizes cholesterol and prevents atherosclerosis. J Hepatol 2019;70:1170-9. [Crossref] [PubMed]
- Caron J, Pene V, Tolosa L, et al. Low-density lipoprotein receptor-deficient hepatocytes differentiated from induced pluripotent stem cells allow familial hypercholesterolemia modeling, CRISPR/Cas-mediated genetic correction, and productive hepatitis C virus infection. Stem Cell Res Ther 2019;10:221. [Crossref] [PubMed]
- Brinkert F, Pukite I, Krebs-Schmitt D, et al. Allogeneic haematopoietic stem cell transplantation eliminates alloreactive inhibitory antibodies after liver transplantation for bile salt export pump deficiency. J Hepatol 2018;69:961-5. [Crossref] [PubMed]
- Bilir BM, Guinette D, Karrer F, et al. Hepatocyte transplantation in acute liver failure. Liver Transpl 2000;6:32-40. [Crossref] [PubMed]
- Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation 2006;82:441-9. [Crossref] [PubMed]
- Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 1999;19:39-48. [Crossref] [PubMed]
- Pareja E, Gomez-Lechon MJ, Cortes M, et al. Human hepatocyte transplantation in patients with hepatic failure awaiting a graft. Eur Surg Res 2013;50:273-81. [Crossref] [PubMed]
- He YT, Qi YN, Zhang BQ, et al. Bioartificial liver support systems for acute liver failure: A systematic review and meta-analysis of the clinical and preclinical literature. World J Gastroenterol 2019;25:3634-48. [Crossref] [PubMed]
- Starkey Lewis PJ, Moroni F, Forbes SJ. Macrophages as a Cell-Based Therapy for Liver Disease. Semin Liver Dis 2019;39:442-51. [Crossref] [PubMed]
- Li YH, Xu Y, Wu HM, et al. Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation in Hepatitis B Virus Related Acute-on-Chronic Liver Failure Treated with Plasma Exchange and Entecavir: a 24-Month Prospective Study. Stem Cell Rev 2016;12:645-53. [Crossref] [PubMed]
- Habeeb MA, Vishwakarma SK, Bardia A, et al. Hepatic stem cells: A viable approach for the treatment of liver cirrhosis. World J Stem Cells 2015;7:859-65. [Crossref] [PubMed]
- Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc 1992;24:3052-3. [PubMed]
- Liang J, Zhang H, Zhao C, et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int J Rheum Dis 2017;20:1219-26. [Crossref] [PubMed]
- Hansel MC, Gramignoli R, Skvorak KJ, et al. The history and use of human hepatocytes for the treatment of liver diseases: the first 100 patients. Curr Protoc Toxicol 2014;62:14.12.1-23.
- Jorns C, Ellis EC, Nowak G, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med 2012;272:201-23. [Crossref] [PubMed]
- Lai YH, Duan WD, Yu Q, et al. Outcomes of liver transplantation for end-stage biliary disease: A comparative study with end-stage liver disease. World J Gastroenterol 2015;21:6296-303. [Crossref] [PubMed]
- Pais R, Barritt AS 4th, Calmus Y, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol 2016;65:1245-57. [Crossref] [PubMed]


