
ADAMTS12 acts as a cancer promoter in colorectal cancer via activating the Wnt/β-catenin signaling pathway in vitro
Introduction
Colorectal cancer (CRC) is ranked as one of most prevalent malignant neoplasms in the digestive system (1). In 2017, it was responsible for about 19.0 million disability-adjusted life-years (DALYs), 1.8 million newly diagnosed cases, and 896,000 cancer-associated deaths (2). Currently, key interventions to the high burden of CRC have been adopted which include the removal of polyps and early diagnosis, and these have been reported to improve prognosis and prolong survival (3). However, the treatment and prevention of CRC is still a global challenge due to the dynamic genomic alternations during CRC progression. For these reasons, the identification of critical molecules in CRC is urgently needed to improve treatment.
ADAMTS12 is a member of the ADAMTS family and contains 19 genes encoding zinc metalloproteinase in mammals. First, described in 1997, this protease family is a group of extracellular, multifunctional enzymes, which may function as a critical regulatory factor implicated in the reciprocal interactions of cells, or cells with extracellular matrices (4,5). So far, numerous studies have demonstrated that ADAMTS metalloproteinase participates in diverse physiological and physiopathological processes in human acquired disorders such as osteoarthritis (6), cardiovascular disease (7), platelet coagulopathy (8), and cancer (9). In cancer-associated progression, ADAMTS12 shows a dual pro or/and anti-tumoral role in proteolytic or non-proteolytic ways. In renal cell carcinoma, ADAMTS12 is listed as 1 of 7 extracellular matrix (ECM) genes, and upregulated and high expression of ADAMTS12 may contribute to metastases (10). The pro-tumoral function has also been demonstrated in human choriocarcinoma JEG-3 cells with the enhancement of tumor invasion in the ADAMTS12-overexpressing cell line (11). Inversely, in vitro and in vivo findings have demonstrated that Adamts12-deficiency displays an increasing angiogenic response and an enhanced tumor invasion, suggesting that ADAMTS12 is a potential antitumogenic factor (12). In CRC, ADAMTS12 has been reported as a factor affecting prognosis of patients (13,14), and epigenetic silence of ADAMTS12 may promote the proliferative properties of tumor cells (15). However, the concrete function and underlying mechanism that ADAMTS12 exerts in CRC have not been sufficiently clarified.
Herein, we are first to report that ADAMTS12 is increased in CRC and, through an analysis of The Cancer Genome Atlas (TCGA) data, we discovered that the increased expression of ADAMTS12 is related to worse prognosis. Mechanically, we found that ADAMTS12 influenced CRC proliferation and migration by targeting Wnt/β-catenin signaling in vitro. Thus, our findings highlight the critical role of ADAMTS-12 for CRC progression and suggest that ADAMTS-12 is a potential therapeutic target for CRC patients.
Methods
Reagents, antibodies, and cell lines
Anti-ADAMTS12, anti-flag, anti-GAPDH, and anti-rabbit horseradish peroxidase (HRP) secondary antibodies were obtained from indicated companies. A β-catenin gene luciferase reporter plasmid was constructed according to the standard molecular biology experiments and applied to determine which signaling pathway the candidate gene modulated. HEK293T and HCT116 cell lines were kept in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U penicillin/streptomycin in standard conditions. All cell lines were purchased from TCGA.
Constructing plasmids and transfection
To obtain Flag-ADAMTS12, cDNA-encoding ADAMTS12 was cloned into a p3×FLAG-CMV-10 vector. An ADAMTS12 knockout plasmid was established by CRISPR/Cas9 hitKO technology. Transient transfection was completed by Lipofectamine 2000 according to the manufacturer’s instructions. The post-transfected cells were incubated and harvested for subsequent assays.
Immunohistochemical analysis
To validate the expression of ADAMTS12 in CRC tissues, we purchased a CRC microarray that included tissues from 41 cancer patients and 41 paracancerous tissues from Wuhan Aiwei Biotechnology Co., Ltd. and carried out an immunohistochemical assay with EnVision kit. Briefly, the deparaffinized and rehydrated slides were pretreated with 0.3% hydrogen peroxidase, followed by blockage with 1% bovine serum albumin. Next, the slides were incubated successively with anti- ADAMTS12, the second antibody, and 3,3-diaminobenzidine (DAB). Finally, HIC staining was performed with Mayer’s hematoxylin before visualization.
Western blot analysis
To confirm the overexpression of indicated proteins, the total cell lysates were harvested, and the protein concentration was determined by Bradford assay. Equivalent protein was resolved by SDS-PAGE and transferred to PVDF membranes. After being placed in blocking buffer for 1 h, the membrane was blotted by the matched antibody for 24 h at 4 °C, followed by secondary antibodies for 1 h. The blotted protein was visualized by HRP Color Development Solution.
Cell proliferation assay
Cell count KIT-8 assay was performed to determine the proliferative capacity of ADAMS12-overexpressing or -deficient HCT116 cells, and 2×103 cells were cultured in a 96-well plate at 37 °C with the 450 nm absorption being recorded every 24 h. Experimental procedures were performed in triplicate.
Clonogenic assay
A colony formation experiment was used to examine the clonogenic potency of tumor cells. The cells were harvested by trypsinization, detached by pipette and quantified using a hemocytometer. Next, 1×103 seed cells were incubated in dishes at 5% CO2 and 37 °C for 10 days. The colonies were counted with a stereomicroscope after fixation and staining.
In vitro cell migration assay
The motility of metastatic cancer cells was examined in a 24-well format transwell apparatus with an 8 µm pore size insert. After trypsinization, the transfected cells were suspended in 200 µL serum-free DMEM and seeded in the upper compartment. Next, the transwell inserts were added to the lower compartment containing DMEM with 0.5% FBS. After incubation at 37 °C and 5% CO2 for 10 h, the insert was taken out and the remaining cell debris was removed with a cotton swab. The cells on the lower side of the insert were counted under a microscope after fixation and cystal violet staining.
Luciferase reporter assays
Since Wnt/β-catenin signaling is closely associated with CRC progression, we examined the impact of the ADAMTS12 expression to the transcriptional activity of b-catenin by luciferase reporter assays (16). HEK293T was transfected with ADAMTS12 expression plasmids or control plasmids and subsequently cotransfected with the β-catenin luciferase reporter vectors and pR-TK control vectors via Lipofectamine 2000. Then, 36 h after transfection, the cells were harvested and prepared so they could go undergo detection for changes in reporter luciferase activities.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
To further confirm the signaling pathway the protein directly modulated, we carried out real-time quantitative polymerase chain reaction (RT-qPCR) to examine the classical downstream target genes of Wnt/β-catenin signaling cascade. The cells were lysed with Trizol reagent. Total RNA was extracted and reverse transcribed according to a traditional molecule clone protocol. The expression level of myc and cyclin D1 was measured with the 2−△△Ct method.
Statistical analysis
GraphPad Prism 7.0 Windows was used for all analyses. For continuous variables, the values are expressed as mean ± SEM. Unpaired two-tailed Student’s t-test was carried out to compare the differences of the ADAMTS12 expression level between tumor tissues and the matched normal adjacent tissues. Prognostic values of colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) patients were analyzed by TCGA database. The viability and migration capacity of the investigated cells were subjected to Mann-Whitney U test. Spearman’s correlation analysis was performed to examine the ADAMTS12 and Wnt/β-catenin signaling pathway. One-way analysis of variance (ANOVA) was applied to compare the difference of the key gene expression level in Wnt/β-catenin signaling pathway. A P value of less than 0.05 was regarded as significant.
Results
Overexpression of ADAMTS-12 in colorectal carcinoma
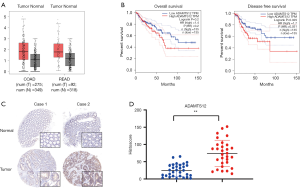
Firstly, we extracted and analyzed the data of CRC and the corresponding adjacent normal tissues from TCGA database. As illustrated in Figure 1A, the mRNA expression of ADAMTS12 in tumor tissues was significantly elevated in comparison with that in their matched benign samples. Kaplan-Meier analysis was further employed to verify the prognostic role of ADAMTS12 expression. The results showed that the overexpression of ADAMTS12 corresponded to the unfavorable prognosis, while no significant association was found between its expression and COAD prognosis (Figure 1B). In addition, a tissue microarray consisting of 41 colorectal tissue samples was adopted to examine the expression of ADAMTS12. Figure 1C illustrates the remarkably increased expression of ADAMTS12 in tumor tissues, which is consistent with the result of TCGA analysis. All the above findings suggest that ADAMTS12 was increased in COAD and READ tissues and may play a pro-oncogenic role in COAD and READ progression.
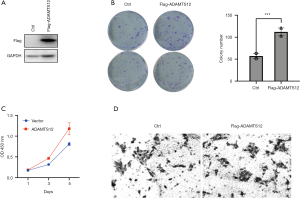
Ectopic ADAMTS-12 expression promotes tumor proliferation and migration in vitro
To further determine the tumorigenic properties of ADAMTS12, we carried out a series of in vitro assays in cells with stable ectopic expression of ADAMTS12. We constructed a flagged ADAMTS12 expression plasmid and transfected it into HCT116 cells, with western blot being used to validate the transfection efficiency with flag-antibody (Figure 2A). Using CCK8 and colony formation assay, we found that overexpression of ADAMTS12 in HCT116 cells significantly promoted the cell viability in comparison with parent cells (Figure 2B,C). In addition, transwell assay was used to test the influence of ADAMTS-12 to the migratory activity of HCT116 cells. In cells of overexpressed ADAMTS12, we also observed a marked increase in migratory potency when compared with the parental cells (Figure 2D). These data suggest that overexpression of ADAMTS12 significantly increase colon cell proliferation and migration.
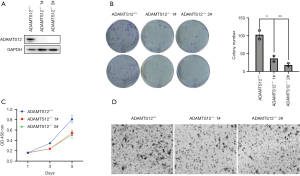
ADAMTS12 deficiency restrains tumor growth and migratory activity in vitro
On account of the obvious overexpression of ADAMTS12 in CRC, we used the HCT116 cell lines to construct ADAMTS12-deficient cells by CRISPR/Cas9 hitKO technology. Western blot was performed for knockout validation (Figure 3A). In the ADAMTS12-deficient background, the colony formation capacity was remarkably reduced, and the number of migratory cells displayed a noticeable decrease (Figure 3B,C,D). Taken together, the genetic inactivation of ADAMTS12 significantly relieved the malignant phenotypes of HCT116 cells.
ADAMTS12 activates Wnt/β-catenin signaling by targeting β-catenin
Subsequently, we implemented a luciferase reporter assay to assess the potential changes in the wnt/β-catenin signaling cascades that ADAMTS12 might have affected in HEK239 cells. As illustrated in Figure 4A, the transcriptional activity of Wnt/β-catenin was stimulated significantly in ADAMTS12-overexpressing cell lines. To confirm the modulation of ADAMTS12 in Wnt/β-catenin signaling cascade, we subsequently examined the key downstream molecules in the Wnt/β-catenin signaling event. As seen in Figure 4B,C,D, myc and cyclin D1, 2 classical target genes of Wnt/β-catenin, were dramatically decreased in ADAMTS12 knockdown HCT116 cell lines, suggesting that ADAMTS12 might be implicated in the malignancy of CRC by directly modulating Wnt/β-catenin signaling pathway.

Discussion
In recent years, several studies have suggested that ADAMTS12 plays a dual pro or/and anti-tumoral role in cancer progression, including in tumorigenesis. However, the precise mechanisms of ADAMTS12 in CRC have not yet been fully understood. Here, we underscored the pro-tumor role of ADAMTS12 in CRC in vitro. Subsequent mechanical findings suggest that ADAMTS12 promoted the viability and migratory behaviors of CRC cells via Wnt1/β-catenin signaling pathway.
ADAMTS12, a secreted metalloproteinase, is a critical regulator in key ECM remodeling events related to tumor malignant behaviors and its aberrant expression is associated with numerous diseases including cancer (12,17). However, its role in CRC is much less understood. First, we compared the ADAMTS12 expression in CRC tissues and its paired adjacent normal tissues by analyzing the data from TCGA and hydrophobic interaction chromatograph (HIC) assays, and the results showed that ADAMTS12 expression was significantly upregulated in tumor tissues. In this regard, our findings were consistent with those of Moncada-Pazos et al. (13), who pointed out that ADAMTS12 gene was activated in stroma surrounding malignant cells. Next, we carried out Kaplan-Meier survival analysis using the download data, and the results revealed that the high expression of ADAMTS12 was relevant with shorter overall survival (OS) and disease-free survival (DFS), suggesting that ADAMTS12 may serve as an unfavorable prognostic indicator for CRC progression. Yet, these results were contrary to a report from Wang and his colleagues. They tested the ADAMTS12 expression of 112 cases of CRC tissues by immunohistochemical staining (14), and their results demonstrated that absence of ADAMTS12 expression in CRC tissues was a poor indicator for CRC patients, indicating ADAMTS12 may serve as a tumor-protective role in CRC progression. A possible explanation for these differences are that the stroma can induce a pro-tumor-anti-tumor response switch when interacting with the neoplastic cells (18).
To further explore the concrete role of ADAMTS12 in CRC progression, we employed a series of in vitro function assays. The results showed that exogenous expression of ADAMTS12 could remarkably promote CRC cell proliferation and migration while silencing ADAMTS12 via CRISPR/Cas9 hitKO technology impaired the proliferative and migratory activity of CRC cells. It is universally known that activating sustained proliferation and metastasis are the typical hallmarks of cancers. As for ADAMTS, evidence has shown that these metalloproteinases interact with cells and ECM-related factors to functionally influence tumor cells migration, proliferation, and invasion. For example, ADAMTS12 was upregulated in invasive human trophoblastic cells while in poorly invasive JEG-3 cells the overexpression of ADAMTS12 could enhance the invasion (11). Accordingly, ADAMTS12 may function as a pro-tumor factor in CRC development and progression in vivo.
Numerous previously published reports have also highlighted the importance of Wnt/β-catenin signaling pathway in the CRC progression (16). Aberrant activation of Wnt/β-catenin signaling pathway enhances the expression of myc and cyclin D1, and eventually contributes to the malignant behaviors of tumor cells. We conducted a dual-luciferase reporter gene assay in order to explore the underlying mechanism of ADAMTS12-mediated cancer proliferation and metastasis. These results indicate that ADAMTS12 positively regulates the transcriptional activity of β-catenin, which was reconfirmed by the lower expression of the downstream target genes including myc and cyclin D1 in cells deficient of ADAMTS12. Our findings suggest that, ADAMTS12 mediated the proliferation and migration of CRC cells via Wnt/β-catenin signaling pathway, which is has been shown to have an important impact on epithelial-mesenchymal transformation (EMT) in HCT116 cells (19). On the other hand, ADAMTS has been reported to play a dominant role in degrading ECM components, and modulating migration and angiogenesis, which may be further trigger epithelial-to-mesenchymal transition (EMT) (20). For example, Adamts18 and ADAM15 are known to interact with E-cadherin which is a critical factor to EMT, modulating the malignant phenotypes of tumor cells (21,22). Thus, we speculated that ADAMTS12 may mediate EMT by Wnt/β-catenin signaling pathway to increase colon tumorigenesis. To our knowledge, we are first to report ADAMTS12 exerting its potency in a non-enzymatic way. Although our study supports the association of ADAMTS12 and EMT, the ADAMTS12-regulated mechanism still needs to clarify in further detail.
In addition, previous studies reported that ADAMTS12 was epigenetically silenced in CRC, acting as a tumor-suppressor enzyme in CRC progression (13,15). However, our results verified the contradictory role of ADAMTS12 in CRC cell lines. These results suggest that ADAMTS12 may indeed play a dual role in CRC progression. The dual properties of ADAMTS12 can also be observed in ADAMTS1 and is caused by the equilibrium of different fragments (23). As for ADAMTS12 in colon cells, it may be reinstated with stimulation (13), which may give us an explanation for the switching between the 2 roles. In addition, transcriptional modification of ADAMTS12 may offer us some clues to explain the dual roles in CRC progression (15). Therefore, subsequent studies will need to elucidate the mechanical balance of ADAMTS12’s dual role induced by CRC.
In conclusion, we demonstrated ADAMTS12 is significantly unregulated in CRC tissues and that ADAMTS12-high CRC patients showed poor survival. Based on the overexpression and knockdown experiment in cells, ADAMTS12 influenced the proliferative and migratory potency of CRC cells by Wnt/β-catenin signaling pathway. Our findings offer new insight into ADAMTS12’s role in CRC carcinogenesis, and suggest that ADAMTS12 may be a valuable therapeutic target for CRC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo G, Chen X, Cai X, et al. Inflammation-based markers can predict the prognosis of geriatric patients with metastatic colorectal cancer receiving first-line chemotherapy. Transl Cancer Res 2019;8:1137-47.
- Collaborators GBDCC. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:913-33. [Crossref] [PubMed]
- Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med 2019;7:609.
- Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol 2018;71-72:225-39. [Crossref] [PubMed]
- Rocks N, Paulissen G, El Hour M, et al. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 2008;90:369-79. [Crossref] [PubMed]
- Sharma N, Drobinski P, Kayed A, et al. Inflammation and joint destruction may be linked to the generation of cartilage metabolites of ADAMTS-5 through activation of toll-like receptors. Osteoarthritis Cartilage 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Li H, Zhou Y, Song W, et al. Expression of ADAMTS-1 mRNA in myocardium of viral heart disease mice and its clinical significance. Exp Ther Med 2019;17:153-8. [PubMed]
- Geys L, Roose E, Scroyen I, et al. Platelet rescue by macrophage depletion in obese ADAMTS-13-deficient mice at risk of thrombotic thrombocytopenic purpura. J Thromb Haemost 2018;16:150-63. [Crossref] [PubMed]
- Yilmaz E, Melekoglu R, Taskapan C, et al. The investigation of serum levels of ADAMTS 5 and 8 (the A disintegrin and metalloproteinase with thrombospondin motifs) in the etiology of endometrial cancer. J Obstet Gynaecol 2019.1-4. [Epub ahead of print]. [PubMed]
- Ho TH, Serie DJ, Parasramka M, et al. Differential gene expression profiling of matched primary renal cell carcinoma and metastases reveals upregulation of extracellular matrix genes. Ann Oncol 2017;28:604-10. [Crossref] [PubMed]
- Beristain AG, Zhu H, Leung PC. Regulated expression of ADAMTS-12 in human trophoblastic cells: a role for ADAMTS-12 in epithelial cell invasion? PLoS One 2011;6:e18473. [Crossref] [PubMed]
- El Hour M, Moncada-Pazos A, Blacher S, et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene 2010;29:3025-32. [Crossref] [PubMed]
- Moncada-Pazos A, Obaya AJ, Fraga MF, et al. The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J Cell Sci 2009;122:2906-13. [Crossref] [PubMed]
- Wang D, Zhu T, Zhang FB, et al. Expression of ADAMTS12 in colorectal cancer-associated stroma prevents cancer development and is a good prognostic indicator of colorectal cancer. Dig Dis Sci 2011;56:3281-7. [Crossref] [PubMed]
- Zheng S, Lin F, Zhang M, et al. Long non-coding RNA AK001058 regulates tumor growth and angiogenesis in colorectal cancer via methylation of ADAMTS12. Am J Transl Res 2019;11:6117-23. [PubMed]
- Bahrami A, Amerizadeh F. Therapeutic Potential of Targeting Wnt/beta-Catenin Pathway in Treatment of Colorectal Cancer: Rational and Progress. J Cell Biochem 2017;118:1979-83. [Crossref] [PubMed]
- Fontanil T, Rua S, Llamazares M, et al. Interaction between the ADAMTS-12 metalloprotease and fibulin-2 induces tumor-suppressive effects in breast cancer cells. Oncotarget 2014;5:1253-64. [Crossref] [PubMed]
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4:839-49. [Crossref] [PubMed]
- Pan B, Zhang T, Yang W, et al. SNX3 suppresses the migration and invasion of colorectal cancer cells by reversing epithelial-to-mesenchymal transition via the beta-catenin pathway. Oncol Lett 2019;18:5332-40. [PubMed]
- Fontanil T, Mohamedi Y, Cobo T, et al. Novel Associations Within the Tumor Microenvironment: Fibulins Meet ADAMTSs. Front Oncol 2019;9:796. [Crossref] [PubMed]
- Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem 2008;283:18393-401. [Crossref] [PubMed]
- Lu T, Dang S, Zhu R, et al. Adamts18 deficiency promotes colon carcinogenesis by enhancing beta-catenin and p38MAPK/ERK1/2 signaling in the mouse model of AOM/DSS-induced colitis-associated colorectal cancer. Oncotarget 2017;8:18979-90. [PubMed]
- Liu YJ, Xu Y, Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 2006;25:2452-67. [Crossref] [PubMed]


