
Responses of gastric epithelial stem cells and their niche to Helicobacter pylori infection
The anatomy of the stomach
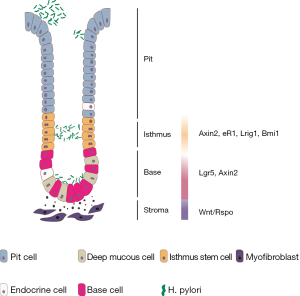
The stomach, as part of the gastrointestinal tract, is an intraperitoneal, muscular, hollow organ located on the left side of the upper abdomen. The gastric mucosa contains a single-layered mucin-generating surface epithelium and specialized cells forming tubular glands. The glands can be divided into the base, the isthmus and the pit facing the lumen (Figure 1). Corpus glands contain short-lived pit and neck mucus cells for mucus production; longer-lived parietal cells, which secrete hydrochloric acid; different hormone-producing enteroendocrine cells, and gastric chief cells, which release pepsinogen and gastric lipase (1,2). In contrast, antral glands have deeper pits and contain fewer differentiated cell types compared to corpus glands, such as surface mucous cells, deep mucous cells, enteroendocrine and tuft cells (1,3). The gastric epithelium is permanently regenerated by a small population of long-lived, dividing stem cells located in the gland itself, which are responsible for constant gland turnover. The turnover kinetics appear to be more rapid in the antrum than in the corpus (4). In general, surface epithelium cells survive only few hours or days, before being shed into the lumen, whereas some cells, such as parietal cells are more long-lived and can survive for weeks or months (5).

Gastric stem cells
Gastric glands are considered monoclonal units originating from self-renewing adult stem cells (6,7). The identification of molecular stem cell markers was recently facilitated by novel lineage tracing techniques in genetic mouse models. These models have significantly expanded our understanding of the gastric gland homeostasis in health and disease (8).
In the antrum, similarly to other parts of the gastrointestinal tract, the leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) has been shown to mark a cell population in the gland base that repopulates full glands. Approximately 30% of Lgr5+ cells are actively cycling. In vivo lineage tracing revealed that the entire gland can be repopulated from the Lgr5+ cell compartment with appearance of various differentiated cell markers, establishing Lgr5 as an antral stem cell marker (9). The Wnt target gene Axin2 also marks Lgr5+ base cells and further expands to a more rapidly proliferating Lgr5-negative cell population in the lower isthmus. Lineage tracing using Axin2 reporter mice revealed that Axin2+/Lgr5+ cells repopulate the gland more rapidly than Lgr5+ cells. Even upon loss of Lgr5+ cells, the remaining Axin2+ cells repopulate entire glands, including new Lgr5+ cells, within seven days (10).
Further markers have been used to mark isthmus cells in the stomach and it has been shown that they are distinct from gland base Lgr5+ cells. Muscle, intestine and stomach expression 1 (Mist1) was shown to mark multipotent progenitors in the isthmus of antrum and corpus (11,12). In addition to Mist1, sex determining region Y-box 2 (Sox2), cholecystokinin 2 receptor (CCK2R) and leucine-rich alpha-2-glycoprotein 1 (Lrig1) have been used as markers of antral and Sox2 and Lrig1 also of corpus stem cells (13-15). Another recently introduced isthmus stem cell marker in the antrum and the corpus is B cell-specific Moloney murine leukemia virus integration site 1 (Bmi1), which has regeneration potential after irradiation or gastric ulcer formation (16). Moreover, a Runt-related transcription factor 1 (Runx1) enhancer element, eR1 was found to be expressed in the isthmus stem cells in antrum and corpus and in a smaller number in basal chief cells (17).
It has been shown that corpus gland base cells are marked by Troy. These cells are quiescent, differentiated chief cells, but they are able to act as ‘reserve’ stem cells and increase their proliferative activity upon injury (18). Later it was confirmed that Troy overlaps with a Lgr5+ subpopulation of chief cells that regenerate entire glands upon injury (19). Lgr5 exclusively labeled 40% of gastric intrinsic factor (GIF) expressing chief cells. Lgr5-GFP+ chief cells also expressed other stem markers including Mist1 and Sox2. However, in contrast to Lgr5, those markers were expressed in broader compartments throughout the gland (19). In agreement with the in vivo data, Troy+ and Lgr5+ chief cells could be cultured to generate long-lived gastric organoids (18,19).
The exact hierarchy of the cell types summarized above remains elusive and it is likely that the presented genes mark at least partially overlapping cell populations. The isthmus appears to be a critical, highly proliferative stem cell compartment in the stomach, whereas gland base cells that express Lgr5 are rather slow cycling and show features of differentiation. Since Lgr5+ cells have been found to consist of several subpopulations (20), it will be important to understand whether the truly differentiated secretory cells in the base occasionally de-differentiate to repopulate the glands or whether Lgr5-lineage tracing data result from a partial overlap of Lgr5 expression with more proliferative isthmus cells. Concerning the corpus, a recent report by Han et al. based on clonal data and single-cell profiling demonstrated elegantly the compartmentalization into two independent long-lived zones with basal, slow-cycling Troy+ and Lgr5+ stem cells and rapidly cycling isthmus Ki67+ and Stathmin1+ (Stmn1+) stem cells (21). Besides rapid vertical expansion, isthmus stem cells showed a slow drift towards clonality via lateral expansion regulated by intercalating parietal cells that act as physical barriers, and not by stem cell competition alone. Of interest, some of the suspected stem cell markers Sox2, Runx1, Lrig1, Mist1, and Bmi1 showed a very broad expression pattern in single cell RNAseq data (21).
It should further be noted that epithelial stem cell hierarchies appear to be context-dependent and that gastrointestinal epithelia in general appear to have high plasticity, with post-mitotic, differentiated cells maintaining the ability to dedifferentiate or transdifferentiate (22). In the small intestine and colon, this high plasticity is well explored, demonstrating that nearly every cell is able to take over stem cell functions (23-25) and it will be important to further explore how acute and chronic stomach injury alters epithelial hierarchy.
Stem cell niche factors support gastric stem cells
Although stem cells have a distinct location and phenotype, their identity and behavior are largely controlled by extrinsic factors from the stem cell niche, i.e., the local microenvironment surrounding the stem cell compartment (26). Various cells, such as subpopulations of neighboring epithelial cells, stromal myofibroblasts, vascular cells, nerves and immune cells constitute the stem cell niche. The notion that the niche is the determining factor controlling the stem cell state derives from studies in mice in which stem cells were depleted. In the antrum, depletion of Lgr5+ cells lead to a rapid repopulation of gland bases that re-acquire the properties of lost Lgr5+ cells within a short period of time (10). Of note, depletion of Lgr5+ cells in the corpus does have an impact on gland physiology, at least in the long-term, suggesting that in the corpus recovery of Lgr5+ cells upon loss is less robust than in the antrum (19).
While these data have suggested the importance of the stem cell niche, more recent studies have provided molecular insights characterizing signals that are required to shape the stem cell compartment. As described above, expression of the stem cell markers Lgr5, as well as Axin2 and Lrig1, requires canonical Wnt signaling, which is active in the gland base (9). The role of Wnt signaling in the stomach has been reviewed recently (27). R-spondin 3 from stromal myofibroblasts, specifically in the base of the stem cell niche, has been demonstrated to be essential for fully active Wnt signaling in the stomach, stem cell identity and epithelial turnover in the antrum (10). While expression of the Wnt target genes Lgr5 and Troy in the corpus gland base implies active Wnt signaling in this compartment, a detailed spatial mapping of Wnt ligands has not been performed for the corpus.
A critical role of Wnt5a deriving from innate lymphoid cells for development of gastric cancer in the corpus has been demonstrated, proving evidence for the contribution of non-canonical Wnt signaling in gastric pathology (11). In addition, a recent report has demonstrated that different Wnt target genes are differentially expressed in Lgr5+ cells in the corpus upon tamoxifen-induced injury (19).
In addition to Wnt signaling, Notch is an important signaling pathway in the gastrointestinal tract, which stimulates stem cell proliferation via activation of the NOTCH1 (N1) and NOTCH2 (N2) receptors in the antrum as well as in the corpus (28,29). Most likely, this pathway functions via direct ligand-receptor interaction between neighboring epithelial cells, since both ligand and receptor are membrane-bound. A detailed review of Notch signaling has recently been published (30). So far, the gastric Notch ligands and their cellular origins remain elusive. However, the functions of Notch receptors have been investigated. Notch inhibition, either globally using a pan-Notch inhibitor or by specific inhibition of N1 and/or N2, disturbed stem cell proliferation in vivo and in organoids. Further, Notch inhibition leads to an expansion of differentiated cells. In the corpus, an increase only of mucous neck-zymogenic lineages were described, while in the antrum mucous and endocrine cells are increased, probably due to the shorter lifespan of cells in the antrum. Conversely, Notch activation yields profound proliferation and reduced differentiation (28,29). In the antrum, Notch activates proliferation of Lgr5+ stem cells and decreases differentiation (31). Inhibition of Notch receptors results in reduced proliferation of antral Lgr5+ stem cells (29). In the corpus, N1 and N2 are expressed by isthmus stem cells, while only N2 is also expressed in the gland base. Both receptors are thought to have an additive function regarding regulation of proliferation (28). Although Wnt and Notch have partially overlapping targets, the exact interplay between both pathways in the stomach is not fully understood.
Bone morphogenetic protein (BMP) signaling also regulates gastric epithelial cell growth and differentiation. Binding of BMP to its receptors leads to the activation of Smad proteins, which enter the nucleus and regulate gene expression. In the stomach BMP-4 stimulates expression of the H+/K+-ATPase α-subunit in parietal cells and enhances gastric acid production, suggesting that BMP signaling induces differentiation into parietal cells (32). Overexpression of Noggin, a secreted factor that inhibits BMP signaling, causes loss of parietal cells and activates proliferation and expansion of transitional cells expressing markers of mucus neck-zymogenic lineages [trefoil factor 2 (Tff2), mucin 6 (Muc6), Griffonia simplicifolia lectin II (GSII)] (33). Noggin thereby induces extracellular signal-regulated kinase (ERK) activation, which contributes to the hyperproliferative state, while loss of parietal cells leads to reduced acid secretion and hypergastrinemia (33). Moreover, it has been demonstrated that inhibition of BMP signaling drives an expansion of Lgr5+ cells (34).
While these studies provide insights into a role of BMP signaling in stem cell regulation, they mostly rely on exogenous manipulation of signaling pathways, such as overexpression of Noggin, which does not show a high level of expression in the murine stomach (own unpublished data). Therefore, it will be important to further explore how the BMP pathway is regulated in the stomach in the context of homeostatic gland turnover and during epithelial injury.
H. pylori initiates changes in gastric epithelial turnover and leads to epithelial pathology
The gram-negative, spiral-shaped bacterium Helicobacter pylori (H. pylori) colonizes about 50% of the world’s population. H. pylori colonizes exclusively the gastric mucosa, usually per oral transmission in early childhood, and persists for life, withstanding gastric acid and immune surveillance (35-38). In most cases colonization with H. pylori remains asymptomatic; however H. pylori induces an active gastritis and is the main risk factor for development of gastroduodenal ulcers and, as a long-term complication, gastric cancer (39). The WHO classifies H. pylori as a class 1 carcinogen. Gastric cancer causes more than 700,000 deaths per year worldwide. Until now a successful vaccine is not available, probably due to bacterial mechanisms of immuno-evasion allowing persistence of H. pylori in its niche (40).
H. pylori survives in the lumen of or in close proximity to gastric glands, where the mucus protects it from the low gastric luminal pH (Figure 2). H. pylori uses a complex motility and chemotaxis system to reach the gastric epithelium. It orientates with four chemoreceptors, namely transducer like proteins (TlpA, TlpB, TlpC, and TlpD) sensing numerous signals such as urea, amino acids, and metals (36,41). Chemoreceptors activate a signal transduction with the Che family that controls the flagellar direction, while the flagellar motor is controlled by the Mot family (42,43). Chemotaxis is important to sense the epithelium and to establish gland colonization, allowing H. pylori to directly interact with stem cells, while Che− mutants are not able to reach stem cells (44,45). Once colonized, multiple bacterial adhesins including blood group antigen binding adhesion (BabA) (46), sialic acid binding adhesion (SabA) (47), Helicobacter outer membrane protein Z (HopZ) (48), outer inflammatory protein A (OipA) (49) and the adherence associated lipoproteins A and B (AlpA/B) (50) contribute the attachment of H. pylori to the epithelium, which is important for the ability of H. pylori to manipulate cell behavior either via direct injection of virulence factors or via secretion of toxins (51).
Among the virulence factors, the cag pathogenicity island (cagPAI) that encodes for the type IV secretion system (T4SS) and cytotoxin-associated gene A (CagA) represents the most prominent and well-studied system. T4SS allows the injection of CagA protein into infected cells, inducing a variety of cellular responses. CagA also allows bacteria to extract nutrients from gastric cells, for example it transfers transferrin receptors from the basolateral membrane to the apical surface where the bacterium locates. Subsequently, H. pylori acquires iron from the host (52). Low host iron levels increase colonization of gastric glands as well as the number of T4SS pili enhancing CagA signaling (53). Both, experimental and clinical data have linked CagA to gastric cancer (54,55).
New insights have revealed that in addition to CagA, ADP-glycero-β-D-manno-heptose (ADP heptose), a small carbohydrate precursor molecule of lipopolysaccharides (LPS) synthesis, is also released into the epithelium via the T4SS. There it binds the alpha-protein kinase 1 (ALPK1) receptor, leading to activation of TRAF-interacting protein with forkhead-associated domain (TIFA) followed by NF-κB activation and the expression of pro-inflammatory target genes such as IL-8Jeny (56-59), establishing a new CagA-independent function of the T4SS.
In addition to aberrant signaling events, direct genotoxic effects of H. pylori have been proposed. By direct contact with epithelial cells, stabilized by the above-mentioned adhesion molecules, H. pylori has been shown to induce DNA double-strand breaks (DSBs) (60). Later it was described that infection with H. pylori containing a functional cagPAI impairs DNA damage response (DDR). This leads to DNA damage preferentially in telomere-proximal, actively transcribed regions. Those susceptible genomic regions show overlaps with gastric cancer genomic aberrations, indicating that some genomic events in gastric cancer could be directly caused by H. pylori infection (61). Moreover, it has been shown, that in stomachs of H. pylori-infected patients, Lgr5+ stem cells show DNA damage linked to oxidative stress (62).
Given the fact that H. pylori can directly induce DNA damage and that it directly interacts with gland base cells in humans and mice, it will be important to address to what extent stem cell DNA damage is driven by direct effects of bacteria versus by more indirect effects such as inflammatory responses. Furthermore, a link between DNA damage and accumulation of mutations in the context of H. pylori has to be further substantiated.
In addition to inducing aberrant cellular signaling via T4SS, a direct interaction of epithelial cells with H. pylori has been shown to modulate immune responses to infection. One key mechanism to evade the immune response is provided by the bacterial factors vacuolating cytotoxin A (VacA) and γ-glutamyl transpeptidase (GGT), which suppress T helper cell activation along with an increase of a regulatory T cells (Tregs) (63,64). A recent report demonstrates an additional mechanism of how direct interaction between epithelial cells and H. pylori may be relevant for immune evasion: H. pylori cholesterol-α-glucosyltransferase (CGT), which extracts cholesterol from the epithelial surface upon attachment, leads to a disturbance of lipid rafts that are required for signal transduction of various proinflammatory cytokines such as IFNγ, IFNβ, IL-6 or IL-22 (65). In this way H. pylori prevents the activation of pro-inflammatory pathways in epithelial cells, thereby blocking epithelial self-defense mechanisms such as secretion of antimicrobial proteins including human beta defensin 3 (hDB3), which has been shown to be induced by IFNγ and to be highly efficient in killing H. pylori (66).
Together, these data illuminate the critical role of direct interaction of H. pylori with epithelial cells, both for creating a protective niche for the bacteria and for inducing cascades that result in gastric disorders. Of note, most of the reports that are summarized above did not take into consideration the aspects of epithelial turnover dynamics that are discussed in the first part of the review, such as the different survival times of cells within a gland. This appears important, as injury of long-lived stem cells might have different impact on the tissue integrity than injury of a cell that will be shed into the lumen within the next hours or days. In addition to consequences of injury, it is important to address whether the biogeography of infection is linked to specific immune responses. Indeed, it has been demonstrated that undifferentiated organoid cultures appear to induce stronger pro-inflammatory responses to H. pylori than differentiated cultures (67,68).
Despite this, for many years the biogeography of H. pylori infection has not been extensively studied. We have been able to apply confocal microscopy and 3-dimensional image reconstruction to demonstrate that indeed a subpopulation of H. pylori are able to colonize the base and isthmus of gastric glands in a mouse model, as well as in human samples. Our techniques allowed a quantification of gland associated H. pylori, showing that approximately half of antral glands were colonized with H. pylori (44). Inside the gland H. pylori preferentially formed microcolonies in the isthmus, containing proliferating progenitor cells, while some bacteria also colonized the glands base (44,69) (Figure 2). In the proliferative zone, bacteria were found in direct contact with mitotic progenitor cells forming extracellular microcolonies directly at epithelial tight junctions. The importance of gland colonization compared to luminal surface colonization for the induction of pathologic changes was demonstrated in C57Bl6 mice from different vendors, where H. pylori in mice from one vendor only colonized antral glands, but not corpus glands, whereas in mice from the second vendor glands were colonized in both, corpus and antrum. It is not entirely clear which environmental factors controlled the differences in gland colonization of the corpus, but after 2 months of infection pathology was observed in sites where gland colonization occurred: while all mice showed antral pathology, only mice with corpus gland colonization showed corpus pathology.
Lineage tracing from cells derived from Lgr5+ stem cells along with hyperplasia was locally accelerated in infected glands compared to uninfected glands in the same animal or in uninfected mice. Thereby the number of bacteria in individual glands correlated positively with the lineage tracing kinetics, indicating that gland-associated H. pylori induced gland turnover. These effects were not observed when using bacteria that are unable to colonize gastric glands and were restricted to the surface mucus due to a mutation in the chemotaxis machinery, demonstrating that the gland turnover is induced specifically by gland-associated bacteria. Of note, H. pylori-induced stem cell activation is dependent on a functional T4SS system, as mice with a defective T4SS have reduced lineage tracing and less hyperplasia compared to WT mice. In addition to induced gland proliferation, the stem cell numbers significantly increased after 2 months of H. pylori infection (44).
To further explore the mechanisms of stem cell expansion upon infection, the regulation of Wnt signaling, being the key component for induction of stem cell associated genes, has been investigated in detail. In contrast to the small intestine, where Paneth cells have been shown to provide Wnt ligands to the stem cell compartment, Wnt ligand expression in the antrum was rather broad and none of the Wnt ligands was found exclusively in the gland base. In contrast, R-spondin 3 was found to be specifically expressed in myosin heavy chain (Myh11+) myofibroblasts surrounding the gland base and it has been demonstrated that the expression of R-spondin 3 is increased upon H. pylori infection, driving the expansion of stem cells and resulting in epithelial gland hyperplasia (10) (Figure 2). Depletion of R-spondin 3 in Myh11+ cells leads to a loss of stem cells, and infection of such mice with H. pylori does not lead to the expansion of stem cells observed in WT mice. In addition to its effect as a mitogen driving an expansion of Axin2+ cells, R-spondin 3 is required for differentiation of gland base cells that co-express Lgr5 as well as markers of differentiated cells such as Pepsinogen C and Gif. These cells are able to counterbalance gland colonization by secreting antimicrobial factors such as intelectin-1 (Itln1) and regenerating family member 3 gamma (Reg3g) upon infection. Itln1 from Lgr5+ cells is able to bind and agglutinate H. pylori in a Ca2+-dependent manner, impairing its motility (20).
While such responses counterbalance gland colonization, some bacteria are able to persist in the stomach. A recent study has explored colonization dynamics of H. pylori in the glands using differentially labelled H. pylori and it has been demonstrated that once bacteria occupy a gland, other incoming strains of H. pylori lack the ability to colonize the same gland (70). It will be important to explore whether epithelial antimicrobial responses to gland associated H. pylori contribute to this colonization resistance, therefore providing a competitive advantage for the first strain.
Mouse models of H. pylori lead to evolution of spasmolytic polypeptide-expressing metaplasia (SPEM) as precancerous lesions in the corpus following parietal cell loss. It has been demonstrated that gland colonization, similarly to the antrum, triggers corpus pathology (44). The cellular origin of SPEM is controversial. Early studies revealed a transdifferentiation from chief cells as SPEM origin, while later studies alternatively proposed the origin in regenerative processes initiated by neck progenitor cells (71,72). While the origin of SPEM is unclear and the pathogenesis of SPEM is beyond the scope of this review, it will important to explore whether H. pylori triggers corpus pathology by directly interfering with the physiology of parietal cells, as suggested by previous reports (73), or whether similarly to the antrum, the stem cell niche guides epithelial behavior, leading to enhanced proliferation, impaired parietal cell differentiation and development of premalignant lesions.
Outlook
Gastric stem cells are now well defined by various markers and their behavior has been well characterized. Recent evidence revealed that the perturbation of gland homeostasis by infection with H. pylori has multiple effects on stem cells, overall resulting in an altered epithelial proliferation and differentiation. Stromal cells show responses to infection and it will be important to further explore the identity of such cells and the regulation of their behavior. In this context, the role of the inflammatory response to H. pylori should be taken into consideration, which might act on epithelial cell behaviors, trigger responses of resident stromal cells and facilitate invasion of new stromal cell populations. The study of epithelial behavior upon infection in vivo, using the ‘tissue microbiology’ approach described in this review may provide new insights into the role of virulence factors of H. pylori, including well studied factors such as CagA as well as new factors such as ADP heptose. Moreover, it will be important to further dissect the stem cell niche in the corpus in the context of H. pylori infection and development of infection-driven premalignant lesions.
Acknowledgments
We would like to thank the members of the Sigal lab and the members of the Department of Molecular Biology at Max Planck Institute for Infection Biology for the helpful discussions during the generation of the manuscript and Rike Zietlow for editing the Manuscript.
Funding: MS was funded by the DFG (DFG Si1983/2-1, SI 1983/3-1, SI 1983/4-1). MS was supported by the BIH Clinician Scientist Program.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.178). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. FT serves as the unpaid editorial board member of Annals of Translational Medicine from Aug 2018 to Jul 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee ER, Trasler J, Dwivedi S, et al. Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am J Anat 1982;164:187-207. [Crossref] [PubMed]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 1993;236:259-79. [Crossref] [PubMed]
- Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: IV. Ultrastructure and renewal of gland cells. Am J Anat 1985;172:241-59. [Crossref] [PubMed]
- Kitsanta P, Triantafyllou K, Chatziargyriou M, et al. Gastric mucosa epithelial cell kinetics are differentiated by anatomic site and Helicobacter pylori infection. Dig Dis Sci 2005;50:1087-91. [Crossref] [PubMed]
- Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 1993;236:314-32. [Crossref] [PubMed]
- Thompson M, Fleming KA, Evans DJ, et al. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development 1990;110:477-81. [PubMed]
- Leushacke M, Ng A, Galle J, et al. Lgr5(+) gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep 2013;5:349-56. [Crossref] [PubMed]
- Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology 2011;140:412-24. [Crossref] [PubMed]
- Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25-36. [Crossref] [PubMed]
- Sigal M, Logan CY, Kapalczynska M, et al. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017;548:451-5. [Crossref] [PubMed]
- Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015;28:800-14. [Crossref] [PubMed]
- Sakitani K, Hayakawa Y, Deng H, et al. CXCR4-expressing Mist1(+) progenitors in the gastric antrum contribute to gastric cancer development. Oncotarget 2017;8:111012-25. [Crossref] [PubMed]
- Hayakawa Y, Jin G, Wang H, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 2015;64:544-53. [Crossref] [PubMed]
- Arnold K, Sarkar A, Yram MA, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011;9:317-29. [Crossref] [PubMed]
- Choi E, Lantz TL, Vlacich G, et al. Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut 2018;67:1595-605. [Crossref] [PubMed]
- Yoshioka T, Fukuda A, Araki O, et al. Bmi1 marks gastric stem cells located in the isthmus in mice. J Pathol 2019;248:179-90. [Crossref] [PubMed]
- Matsuo J, Kimura S, Yamamura A, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology 2017;152:218-31.e14. [Crossref] [PubMed]
- Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013;155:357-68. [Crossref] [PubMed]
- Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 2017;19:774-86. [Crossref] [PubMed]
- Sigal M, Reines MDM, Mullerke S, et al. R-spondin-3 induces secretory, antimicrobial Lgr5(+) cells in the stomach. Nat Cell Biol 2019;21:812-23. [Crossref] [PubMed]
- Han S, Fink J, Jorg DJ, et al. Defining the Identity and Dynamics of Adult Gastric Isthmus Stem Cells. Cell Stem Cell 2019;25:342-56.e7. [Crossref] [PubMed]
- Burclaff J, Mills JC. Plasticity of differentiated cells in wound repair and tumorigenesis, part I: stomach and pancreas. Dis Model Mech 2018;11:dmm033373. [Crossref] [PubMed]
- Harnack C, Berger H, Antanaviciute A, et al. R-spondin 3 promotes stem cell recovery and epithelial regeneration in the colon. Nat Commun 2019;10:4368. [Crossref] [PubMed]
- Tetteh PW, Basak O, Farin HF, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell 2016;18:203-13. [Crossref] [PubMed]
- Tomic G, Morrissey E, Kozar S, et al. Phospho-regulation of ATOH1 Is Required for Plasticity of Secretory Progenitors and Tissue Regeneration. Cell Stem Cell 2018;23:436-43.e7. [Crossref] [PubMed]
- Bartfeld S, Koo BK. Adult gastric stem cells and their niches. Wiley Interdiscip Rev Dev Biol 2017. [Crossref] [PubMed]
- Fischer AS, Sigal M. The Role of Wnt and R-spondin in the Stomach During Health and Disease. Biomedicines 2019. [Crossref] [PubMed]
- Demitrack ES, Gifford GB, Keeley TM, et al. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol 2017;312:G133-44. [Crossref] [PubMed]
- Gifford GB, Demitrack ES, Keeley TM, et al. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut 2017;66:1001-11. [Crossref] [PubMed]
- Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol 2016;594:4791-803. [Crossref] [PubMed]
- Demitrack ES, Gifford GB, Keeley TM, et al. Notch signaling regulates gastric antral LGR5 stem cell function. Embo J 2015;34:2522-36. [Crossref] [PubMed]
- Nitsche H, Ramamoorthy S, Sareban M, et al. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am J Physiol Gastrointest Liver Physiol 2007;293:G607-14. [Crossref] [PubMed]
- Shinohara M, Mao M, Keeley TM, et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology 2010;139:2050-60.e2. [Crossref] [PubMed]
- Ye W, Takabayashi H, Yang Y, et al. Regulation of Gastric Lgr5+ve Cell Homeostasis by Bone Morphogenetic Protein (BMP) Signaling and Inflammatory Stimuli. Cell Mol Gastroenterol Hepatol 2018;5:523-38. [Crossref] [PubMed]
- Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420-9. [Crossref] [PubMed]
- Johnson KS, Ottemann KM. Colonization, localization, and inflammation: the roles of H. pylori chemotaxis in vivo. Curr Opin Microbiol 2018;41:51-7. [Crossref] [PubMed]
- Monack DM. Helicobacter and salmonella persistent infection strategies. Cold Spring Harb Perspect Med 2013;3:a010348. [Crossref] [PubMed]
- Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol 2013;11:385-99. [Crossref] [PubMed]
- Bauer B, Meyer TF. The Human Gastric Pathogen Helicobacter pylori and Its Association with Gastric Cancer and Ulcer Disease. Ulcers 2011;2011:1-23.
- Meyer TF, Morey P. Chapter 33 - A Future for a Vaccine Against the Cancer-Inducing Bacterium Helicobacter pylori? In: Kiyono H, Pascual DW. editors. Mucosal Vaccines (Second Edition). Academic Press; 2020:579-96.
- Hanyu H, Engevik KA, Matthis AL, et al. Helicobacter pylori Uses the TlpB Receptor To Sense Sites of Gastric Injury. Infect Immun 2019. [Crossref] [PubMed]
- Howitt MR, Lee JY, Lertsethtakarn P, et al. ChePep controls Helicobacter pylori Infection of the gastric glands and chemotaxis in the Epsilonproteobacteria. mBio 2011. [Crossref] [PubMed]
- Ottemann KM, Lowenthal AC. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect Immun 2002;70:1984-90. [Crossref] [PubMed]
- Sigal M, Rothenberg ME, Logan CY, et al. Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology 2015;148:1392-404.e21. [Crossref] [PubMed]
- Collins KD, Hu S, Grasberger H, et al. Chemotaxis Allows Bacteria To Overcome Host-Generated Reactive Oxygen Species That Constrain Gland Colonization. Infect Immun 2018. [Crossref] [PubMed]
- Ilver D, Arnqvist A, Ogren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 1998;279:373-7. [Crossref] [PubMed]
- Mahdavi J, Sonden B, Hurtig M, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 2002;297:573-8. [Crossref] [PubMed]
- Peck B, Ortkamp M, Diehl KD, et al. Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Res 1999;27:3325-33. [Crossref] [PubMed]
- Yamaoka Y, Kwon DH, Graham DY. A M. (r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A 2000;97:7533-8. [Crossref] [PubMed]
- Odenbreit S, Till M, Hofreuter D, et al. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol 1999;31:1537-48. [Crossref] [PubMed]
- Backert S, Clyne M, Tegtmeyer N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal 2011;9:28. [Crossref] [PubMed]
- Tan S, Noto JM, Romero-Gallo J, et al. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog 2011;7:e1002050. [Crossref] [PubMed]
- Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016;150:64-78. [Crossref] [PubMed]
- Parsonnet J, Friedman GD, Orentreich N, et al. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 1997;40:297-301. [Crossref] [PubMed]
- Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A 2008;105:1003-8. [Crossref] [PubMed]
- Zimmermann S, Pfannkuch L, Al-Zeer MA, et al. ALPK1- and TIFA-Dependent Innate Immune Response Triggered by the Helicobacter pylori Type IV Secretion System. Cell Rep 2017;20:2384-95. [Crossref] [PubMed]
- Pfannkuch L, Hurwitz R, Traulsen J, et al. ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori. FASEB J 2019;33:9087-99. [Crossref] [PubMed]
- Stein SC, Faber E, Bats SH, et al. Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog 2017;13:e1006514. [Crossref] [PubMed]
- Gall A, Gaudet RG, Gray-Owen SD, et al. TIFA Signaling in Gastric Epithelial Cells Initiates the cag Type 4 Secretion System-Dependent Innate Immune Response to Helicobacter pylori Infection. mBio 2017. [Crossref] [PubMed]
- Toller IM, Neelsen KJ, Steger M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A 2011;108:14944-9. [Crossref] [PubMed]
- Koeppel M, Garcia-Alcalde F, Glowinski F, et al. Helicobacter pylori Infection Causes Characteristic DNA Damage Patterns in Human Cells. Cell Rep 2015;11:1703-13. [Crossref] [PubMed]
- Uehara T, Ma D, Yao Y, et al. H. pylori infection is associated with DNA damage of Lgr5-positive epithelial stem cells in the stomach of patients with gastric cancer. Dig Dis Sci 2013;58:140-9. [Crossref] [PubMed]
- Gebert B, Fischer W, Weiss E, et al. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 2003;301:1099-102. [Crossref] [PubMed]
- Oertli M, Noben M, Engler DB, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A 2013;110:3047-52. [Crossref] [PubMed]
- Morey P, Pfannkuch L, Pang E, et al. Helicobacter pylori Depletes Cholesterol in Gastric Glands to Prevent Interferon Gamma Signaling and Escape the Inflammatory Response. Gastroenterology 2018;154:1391-404.e9. [Crossref] [PubMed]
- Bauer B, Pang E, Holland C, et al. The Helicobacter pylori virulence effector CagA abrogates human beta-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe 2012;11:576-86. [Crossref] [PubMed]
- Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015;148:126-36.e6. [Crossref] [PubMed]
- Boccellato F, Woelffling S, Imai-Matsushima A, et al. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut 2018. [Epub ahead of print]. [PubMed]
- Earle KA, Billings G, Sigal M, et al. Quantitative Imaging of Gut Microbiota Spatial Organization. Cell Host Microbe 2015;18:478-88. [Crossref] [PubMed]
- Fung C, Tan S, Nakajima M, et al. High-resolution mapping reveals that microniches in the gastric glands control Helicobacter pylori colonization of the stomach. PLoS Biol 2019;17:e3000231. [Crossref] [PubMed]
- Koulis A, Buckle A, Boussioutas A. Premalignant lesions and gastric cancer: Current understanding. World J Gastrointest Oncol 2019;11:665-78. [Crossref] [PubMed]
- Kinoshita H, Hayakawa Y, Niu Z, et al. Mature gastric chief cells are not required for the development of metaplasia. Am J Physiol Gastrointest Liver Physiol 2018;314:G583-96. [Crossref] [PubMed]
- Yao X, Smolka AJ. Gastric Parietal Cell Physiology and Helicobacter pylori-Induced Disease. Gastroenterology 2019;156:2158-73. [Crossref] [PubMed]


