
X-linked dominant protoporphyria in a Chinese pedigree reveals a four-based deletion of ALAS2
Introduction
Protoporphyria encompasses two clinically similar but distinct hereditary disorders with different inheritance patterns: the more common erythropoietic protoporphyria (EPP, OMIM#177000) and a newly described entity, X-linked dominant protoporphyria (XLDPP, OMIM#300752) (1,2). XLDPP is due to gain-of-function deletions or alteration variants in the C-terminal of the 5'-aminolevulinic acid synthase 2 (ALAS2) gene (1,2). The ALAS2 enzyme is a specific isoform of the first enzyme in the hematopoietic biosynthesis pathway (2). Clinically, the onset of lifelong photosensitivity occurs early in life, such as in the neonatal period. While the dermatological findings of XLDPP are indistinguishable from those of EPP, XLDPP patients more commonly develop liver diseases and typically have higher concentrations of erythrocyte protoporphyrin (2). Protoporphyrin is a hydrophobic molecule secreted by the liver, and for this reason, blood testing of the free protoporphyrin level is instrumental in the diagnosis of protoporphyria. However, the biochemical blood test does not differentiate XLDPP from EPP, which requires genetic analysis. Herein, we describe the clinical and laboratory findings of a multigenerational Chinese pedigree afflicted by XLDPP and report the identification of a pathogenic variant in the ALAS2 gene.
Methods
Ethics statement
This study was performed under the guidance of the principles of the Declaration of Helsinki. Approval was obtained from the ethics committee of Peking Union Medical College Hospital for the collection of patient samples. The family supplied informed consent and understood that all patient samples would be used for research and genetic counseling. All regulations about patient enrollment, sample collection, and informed consent for this research was followed.
Standard blood and serum testing
Routine laboratory examinations, which included routine blood and biochemical tests, were performed using peripheral blood samples collected from patients.
Clinical observation and examination
Specific cutaneous manifestations of the proband and pedigree information were documented. Ultrasound examination was performed to evaluate the liver and gallbladder, while dermatoscopy was performed to evaluate skin aging. Also, very high-frequency skin ultrasonic (VHF-US) examination and Fotofinder system evaluations were performed to evaluate photodamage.
Histopathological examination and staining
A 4-mm punch biopsy of a skin lesion overlying the right superior temple of the proband was performed for routine histological examination. Five-micrometer-thick sections were stained with hematoxylin and eosin (H&E) and periodic acid Schiff (3) for histopathological evaluation.
Genomic DNA extraction
Genomic DNA was extracted from peripheral blood samples of the proband (IV:1), his biological parents (III:1 and III:2), and other relatives (as shown in Figure 1A) using the QIAamp DNA Blood Mini Kit (QIAgen, Hilden, Germany), following the manufacturer’s instructions. Blood samples were processed within 24 h of collection.
Targeted next-generation sequencing (NGS) of porphyria-associated genes
After quality control, 1 µg of genomic DNA was randomly fragmented to ~250 bp using Covaris S2. Next, end repair and A-tailing were performed. Then, adapters were ligated to both ends of the fragments. After purification, the adaptor-ligated DNA fragments were amplified, purified, pooled, and hybridized to a 2.1 M human array (Roche NimbleGen) for enrichment. The array targeted exons and 10-bp flanking intronic sequences of nine porphyria-related genes (ALAS2, ALAD, CPOX, FECH, GATA1, HMBS, PPOX, UROS, and UROD). After library quality control, NGS libraries were then sequenced on Illumina HiSeq2500 to generate paired-end reads. After filtering, all the clean reads were aligned to hg19 using Burrows-Wheeler Aligner (BWA). Small nucleotide variants (SNVs) and insertions/deletions (indels) were detected using SOAPsnp and SamTools.
Confirmation of variants by Sanger sequencing
For variants filtered by the NGS data processing pipeline, Sanger sequencing was used to confirm that they co-segregated with the disease phenotype in the family. Primers were designed (Table 1), and polymerase chain reaction (PCR) was conducted as previously described (4). PCR products were then subjected to Sanger sequencing. The sequencing results were later analyzed using Codon Code Aligner.

Full table
Results
Clinical observations and family history presentation
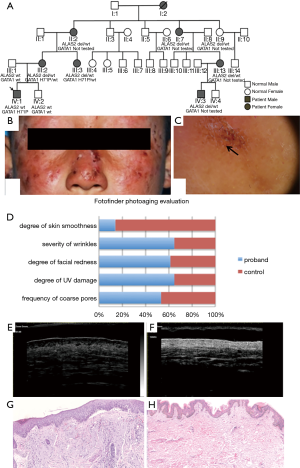
The proband (Figure 1A, IV:1, the first boy in the fourth generation of the family) was a 7-year-old male who presented with facial scarring and a long history of cutaneous photosensitivity with pain, pruritus, erythema, and edema hours after sun exposure. These symptoms had been present since birth. Areas of hemorrhagic crusting and scarring associated with excoriations were also noted. There was no history of erythruria. Physical examination revealed pink, lichenified erythematous macules and thin edematous plaques with overlying hemorrhagic crust and excoriations symmetrically involving the most sun-exposed areas of the nose, cheeks, chin, auricles, dorsal hands, and periorbital skin. Areas of atrophy and varioliform scars were also noted on the affected skin as well as some radial, atrophic furrows involving the perioral skin (Figure 1B). There was no evidence of erythrodontia.
The proband’s mother (III:2), maternal aunt (III:3), maternal grandmother (II:2), maternal great-aunt (II:8), great maternal grandmother (I:2), and maternal cousin (IV:3) exhibited similar dermatological symptoms of varying levels of severity. III:2 had no visible dermatological abnormalities on examination but did report a history of cutaneous pruritus and pain involving the face and hands shortly after sun exposure. Physical examination revealed many pitted scars on the nasal dorsum, reminiscent of atrophoderma vermiculata, along with radial, atrophic, furrowed changes peri-orally. III:3 reported a history of a mild burning sensation of the face shortly after sun exposure, without dermatological sequelae such as scars or blisters. Other female maternal relatives (II:2, II:8, III:13, and IV:3) had similar lesions, whose skin color on examination exhibited a brownish-yellow hue with significant lichenification of the face and dorsal hands, both of which had a thick, rough texture on palpation. The proband’s male 16-year-old maternal second cousin (IV:3) had complained of similar symptoms throughout childhood and adolescence. Upon review of the afflicted pedigree, the inheritance of this disorder was consistent with an X-linked dominant pattern.
Laboratory examination
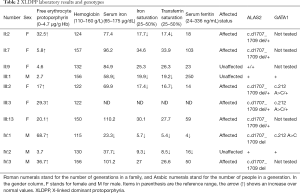
Blood measurements, including of uroporphyrin and hematoporphyrin levels, were performed using peripheral blood samples of the family. Free erythrocyte protoporphyrin levels were significantly elevated in all affected family members and notably higher in both males (the proband, IV:1, and his maternal second cousin, IV:3. Affected family members were also found to have varying degrees of iron deficiency (Table 2).
Full table
Hepatic ultrasound examination
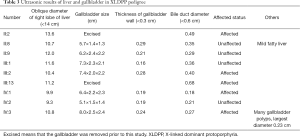
Both family members II:2 and III:3 underwent cholecystectomy. Family member IV:3 underwent an abdominal ultrasound examination, which revealed an enlarged gallbladder having multiple polyps, with a largest diameter of 0.23 cm (Table 3).
Full table
Dermatoscopic examination
The sun-exposed areas of the face, including the temple and nasal dorsum, were examined by dermatoscopy. The proband’s mother’s skin lesions (III:2) showed pink patches and white reticular spots. In contrast, those of the proband (IV:1) exhibited a brownish-yellow background, prominent follicular plugging, perifollicular white halos, scattered brown spotty pigmentation, conspicuous linear and reticular white spots, focal ulceration, and hemorrhagic crust (Figure 1C).
Fotofinder photoaging evaluation
Based on the analysis of the facial skin using the Fotofinder system, the photoaging scores of the proband (Figure 1D) revealed more severe photodamage than that of the control group in this system:
- The frequency of coarse pores was 14% higher than that of the control;
- The degree of UV damage was 85% higher than that of the control;
- The degree of facial redness was 58% higher than that of the control;
- The severity of wrinkles was 83% higher than that of the control;
- The degree of skin smoothness was 84% less than that of the control.
VHF skin ultrasonic examination results
VHF skin ultrasonic examination of the nasal dorsum showed significant epidermal thickening (Figure 1E) compare with the normal control (Figure 1F). Also, diffuse hypoechogenicity in the dermis with no obvious abnormality was seen.
Skin pathology examination
Pathological examination of the H&E-stained skin biopsy showed focal parakeratosis, vacuolated cells in the epidermis, and many foci of sub-epidermal splitting. Besides, epidermal acanthosis, thickened collagen bundles, a clear basophilic pattern, structural disorder of the papillary dermis, capillary dilation, and hypervascularity were present (Figure 1G). That is severe photodamaging changes than normal control (Figure 1H).
Genetic analysis results
Targeted NGS of nine porphyria-related genes identified the ALAS2 (NM_000032.4): c.1706_1709delAGTG/p.(E569fs), ChrX:g.55,035,668_55,035,671del(hg19) a variant in the hemizygous state in the proband (Figure 2A). Sanger sequencing confirmed that the variant was hemizygous in the proband (IV:1) and co-segregated with the disease in the hemizygous state in his affected male second cousin (IV:3). The same genetic variant was also found in the heterozygous state in five affected female relatives (II:2, II:8, III:2, III:3, and III:13). This variant was not found in three unaffected family members evaluated (II:9, III:1, and IV:2) (Table 2) (Figure 1A). Because I:1 was known to be unaffected and the deceased I:2 had been affected, I:2 was deduced to have been an obligate carrier of the variant.
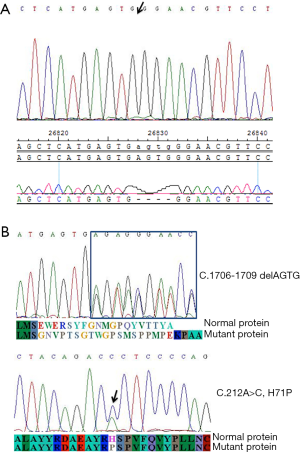
A missense variant in GATA1 (NM_002049.3), c.212A>C, p.(H71P), ChrX:g.48,649,728A>C(hg19), was also identified in the hemizygous state in the proband (IV:1). Sanger sequencing confirmed that the variant was hemizygous in the proband (IV:1) and heterozygous in both the proband’s mother (III:2) and his maternal aunt (III:3) (Figure 2B).
Discussion
XLDPP mainly affects male patients who typically present with photosensitive paresthesia, burning, pain, and pruritus, followed by erythematous swelling resembling solar urticaria during infancy or early childhood (4) (Figure 3). These symptoms typically begin at once upon sun exposure. Less commonly, patients may present with blisters, which may be excoriated and unroofed, leaving scant hemorrhagic crust and collarettes of scale (5). The cutaneous pain and dermatitis can expand beyond the sun-exposed sites and may last for hours to days (6). The inherited predisposition to photosensitivity persists throughout life and eventually results in chronic actinic damage secondary to repeated attacks. This manifests clinically as a “weather-beaten” appearance, consisting of lichenification, a leather-like skin texture, pseudo-vesicles, perioral radial furrowing, and platonychia or koilonychia. Some patients develop liver disease, but their condition may not result in a reduced life expectancy (7). Fulminant hepatitis may, albeit rarely, develop and is the most severe outcome, potentially progressing to liver failure and liver transplantation (6). The clinical spectrum of heterozygous female carriers ranges from completely asymptomatic to as severely affected as male patients (Figure 3).
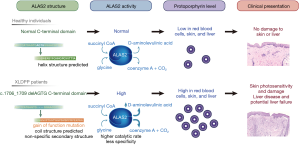
Whatley et al. first reported XLDPP in 2008 (2). Frameshift variants affecting the last 19–20 residues in the C-terminal region of ALAS2 were shown to result in a significant increase in ALAS2 enzyme activity. To date, no more than 50 XLDPP patients have been reported in the literature, none of whom was of Chinese descent. Pathogenic variants in ALAS2 reported in patients with XLDPP include: c.1706_1709delAGTG (p.E569GfsX24), c.1699_1700delAT (p.M567EfsX2), c.1642C>T (p.Q548X), c.1651_1676del (p.S551PfsX5), and c.1737delG (p.Q581SfsX13). All of these variants are predicted to result in premature truncation within the last exon of ALAS2 (1,2,5), which would escape nonsense-mediated decay but lead to a gain-of-function mechanism related to structural alterations of the ALAS2 enzyme C-terminus. Male patients with XLDPP have more severe skin involvement than do their heterozygous female counterparts. This phenomenon can be explained by the random inactivation of the X chromosome (lyonization) (6). Because females have two X chromosomes and one copy becomes randomly inactivated during early embryonic development, the proportion of mutant X-chromosome inactivation may explain phenotypic variability in X-linked dominant genetic diseases. This finding differs from that in an earlier study (7), which found that the proportion of X-chromosome inactivation may differ dramatically and also be associated with significant phenotypic variability.
Since the first report of XLDPP found to be caused by underlying gain-of-function variants in ALAS2, there have been few studies reporting on XLDPP, with most cases occurring in Europe. This article is based on a study first reported in 2016. We here describe the first Chinese XLDPP pedigree affected by this condition (8). The c.1706_1709delAGTG (p. E569GfsX24) variant found through this investigation was previously reported in other XLDPP patients (2,5). In this study, it was present in seven affected family members, and one affected obligate carrier (I:2), but not in three unaffected relatives (2,5). This variant has not been found in extensive population studies. Functional studies have shown that this variant leads to increased ALAS2 activity, which handles the accumulation of protoporphyrin (2).
In summary, this variant meets the criteria to be classified as pathogenic based on its recurrence in multiple unrelated affected probands, co-segregation with the phenotype among family members, and the functional impact of the protein, according to the professional standards and guidelines recommended by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (9). The male proband and his maternal male second cousin developed more severe symptoms than did female relatives. However, with increasing age, female patients had developed the hepatobiliary disease (cholestasis) requiring gallbladder removal. These findings suggest an increased risk of gallbladder disease, which is a known complication associated with XLDPP (Table 3).
In the present study, affected individuals were also found to have iron deficiency, which has not been reported in earlier cases (2). During erythropoiesis, the first step of heme biosynthesis is the synthesis of ALA by succinyl coenzyme A and glycine. Erythrocyte system-specific ALAS2 is a critical rate-limiting enzyme for this synthesis. Of interest, we additionally named a missense variant in GATA1 in this family. GATA1 is one of the six members of the GATA gene family, encoding a group of related transcription factors discovered in the 1980s (10). Defects cause six rare syndromes in GATA1 gene expression or abnormal protein production: X-linked thrombocytopenia (XLT), X-linked thrombocytopenia with thalassemia (XLTT), congenital erythropoietic porphyria (CEP), transient myeloproliferative disorder (TMD), acute megakaryoblastic leukemia (AMKL) associated with Trisomy 21, and X-linked anemia with or without neutropenia and/or platelet abnormalities (XLANP). CEP is a rare variant of porphyria. Patients with CEP also present with early-onset cutaneous photosensitivity and increased porphyrin derivatives uroporphyrin I and coproporphyrin I isomers due to decreased uroporphyrinogen-synthase (UROS) activity. Biallelic pathogenic variants in the UROS gene that encodes the enzyme cause autosomal recessive CEP. In addition, a pathogenic variant in GATA1 on the X chromosome is associated with impaired erythropoiesis (11) and CEP (12). Furthermore, several studies found that CEP may be associated with underlying mutations in GATA1 (12-14). Mouse models suggest that the GATA1 short isoform supports normal adult megakaryopoiesis, platelet formation, and erythropoiesis, which is consistent with its dysfunction in the conditions noted above. A splice site variant in GATA1, c.220+1G>C flanking exon 2, was also demonstrated to lead to the synthesis of only the short isoform of GATA1 due to exon 2 skipping in seven affected males from two generations of an afflicted pedigree. Hematological profiles of the affected males proved macrocytic anemia, normal platelet counts, and neutropenia in most cases. Altogether, these findings suggest that the GATA1 short isoform alone, produced at low or normal levels, is insufficient to support normal erythropoiesis (11). In our study, the affected individuals (II:2, III:1, III:2, IV:1, IV:2) demonstrated iron deficiency, and the proband had iron deficiency anemia [MCV 79.9 fl (82–97), MCH 25.2 pg (27–32)]. This is a finding distinct from the phenotype reported in patients with GATA1 pathogenic variants. The p.(H71P) variant is in exon 2 and has been found in 14/12,782 East Asian chromosomes including eight hemizygotes, as reported in the Genome Aggregation Database (http://gnomad.broadinstitute.org/variant/X-48649728-A-C). Although the variant has been reported in the general population, the allele frequency is low enough to be consistent with the incidence of anemia in the general population. Multiple computational tools suggest an impact of this variant on the protein, but this information is not sufficiently predictive to determine pathogenicity. In summary, the clinical significance of the p. (H71P) a variant in GATA1 is unclear and added studies are needed to establish the genotype-phenotype correlation.
At present, treatment options for patients with XLDPP are limited, and the painful photosensitivity caused by protoporphyrin IX accumulation is challenging to treat. A new approach for treating these patients is to administer an alfamelanotide implant that is placed under the skin, which performs the controlled release of the synthetic peptide for two months. Alfamelanotide is an analog of alpha-melanocyte-stimulating hormone used to prevent skin damage from the sun in people with protoporphyria. Although it is costly and difficult to access in some countries, it opens up new possibilities for affected patients (15).
In summary, we have described the genetic diagnosis of XLDPP in the first reported multigenerational Chinese pedigree of its kind. The ALAS2 C-terminal frameshift variant caused the condition in the proband and affected family members (Figure 3). Although a GATA1 missense variant was also found in affected individuals with concomitant iron deficiency, the primary clinical and laboratory abnormalities in this study were caused by ALAS2 mutation.
Further analysis of the enzymatic changes caused by ALAS2 mutation lacks in this study. For patients with gallbladder involvement in the family, no further pathological changes of gallbladder and liver were detected. Therefore, this paper is limited to being a report of patients, and more reports are needed to describe XLDPP comprehensively.
Acknowledgments
Funding: This work was supported by grants from the Fundamental Research Funds for the Central Universities (3332018025), NCMI-ABD02-201709, and Beijing Dongcheng District Excellent Talent Support Training project (2019JGM-5). The authors thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed under the guidance of the principles of the Declaration of Helsinki. Approval was obtained from the ethics committee of Peking Union Medical College Hospital for the collection of patient samples. The family supplied informed consent and understood that all patient samples would be used for research and genetic counseling. All regulations about patient enrollment, sample collection, and informed consent for this research was followed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Balwani M, Doheny D, Bishop DF, et al. Loss-of-function ferrochelatase and gain-of-function erythroid-specific 5-aminolevulinate synthase mutations causing erythropoietic protoporphyria and x-linked protoporphyria in North American patients reveal novel mutations and a high prevalence of X-linked protoporphyria. Mol Med 2013;19:26-35. [Crossref] [PubMed]
- Whatley SD, Ducamp S, Gouya L, et al. C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet 2008;83:408-14. [Crossref] [PubMed]
- De Petris G, Chen L, Pasha SF, et al. Cronkhite-Canada syndrome diagnosis in the absence of gastrointestinal polyps: a case report. Int J Surg Pathol 2013;21:627-31. [Crossref] [PubMed]
- Wang T, Xu C, Zhou X, et al. Homozygous ALOXE3 Nonsense Variant Identified in a Patient with Non-Bullous Congenital Ichthyosiform Erythroderma Complicated by Superimposed Bullous Majocchi's Granuloma: The Consequences of Skin Barrier Dysfunction. Int J Mol Sci 2015;16:21791-801. [Crossref] [PubMed]
- Ducamp S, Schneider-Yin X, de Rooij F, et al. Molecular and functional analysis of the C-terminal region of human erythroid-specific 5-aminolevulinic synthase associated with X-linked dominant protoporphyria (XLDPP). Hum Mol Genet 2013;22:1280-8. [Crossref] [PubMed]
- Brancaleoni V, Balwani M, Granata F, et al. X-chromosomal inactivation directly influences the phenotypic manifestation of X-linked protoporphyria. Clin Genet 2016;89:20-6. [Crossref] [PubMed]
- Ninomiya Y, Kokunai Y, Tanizaki H, Akasaka E, Nakano H, Moriwaki S. X-linked dominant protoporphyria: The first reported Japanese case. J Dermatol 2016;43:414-8. [Crossref] [PubMed]
- Wang T, Dong Q, Xu C, et al. X-linked dominant protoporphyria: report of a pedigree and detection of ALAS2 gene mutations. Chinese Journal of Dermatology 2016;49:702-5.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Ciovacco WA, Raskind WH, Kacena MA. Human phenotypes associated with GATA-1 mutations. Gene 2008;427:1-6. [Crossref] [PubMed]
- Hollanda LM, Lima CS, Cunha AF, et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet 2006;38:807-12. [Crossref] [PubMed]
- Phillips JD, Steensma DP, Pulsipher MA, et al. Congenital erythropoietic porphyria due to a mutation in GATA1: the first trans-acting mutation causative for a human porphyria. Blood 2007;109:2618-21. [Crossref] [PubMed]
- Di Pierro E, Russo R, Karakas Z, et al. Congenital erythropoietic porphyria linked to GATA1-R216W mutation: challenges for diagnosis. Eur J Haematol 2015;94:491-7. [Crossref] [PubMed]
- Solis C, Aizencang GI, Astrin KH, et al. Uroporphyrinogen III synthase erythroid promoter mutations in adjacent GATA1 and CP2 elements cause congenital erythropoietic porphyria. J Clin Invest 2001;107:753-62. [Crossref] [PubMed]
- Biolcati G, Marchesini E, Sorge F, et al. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. Br J Dermatol. 2015;172:1601-12. [Crossref] [PubMed]


