Shorter leucocyte telomere length as a potential biomarker for nonalcoholic fatty liver disease-related advanced fibrosis in T2DM patients
Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is rising dramatically worldwide, with approximately 1 billion individuals afflicted globally (1). NAFLD-related advanced fibrosis is considered to be the main risk factor among various disease spectrum of NAFLD (2,3) for all-cause mortality (4), especially for cardiovascular events (5). Patients with type 2 diabetes mellitus (T2DM) have extremely higher risk of NAFLD, which is around 49% to 62% (6,7). T2DM increases the risk of liver-related deaths, as well as overall mortality in NAFLD patients (6), meanwhile increases the risk of cardiovascular disease and accelerates the progression of macro and microvascular complications (8). In addition, patients with NAFLD presenting hepatocellular carcinoma in cirrhotic liver were more likely to be obese or have type 2 diabetes (9). The prevalence of diabetes around the world, especially in China, is getting more challenging in recent years (10). Therefore, screening for NAFLD-related advanced fibrosis should be considered in T2DM patients.
The conventional methods used as diagnostic for liver fibrosis have some limitation. Liver biopsy is the golden standard for liver fibrosis. But liver biopsy is an invasive procedure, along with some complications and sampling error. Therefore, it is essential to identify the biomarker for screening NAFLD-related advanced fibrosis.
Telomere is duplicate sequences of (TTAGGG) that relates to shelterin proteins. Telomere is considered to protect chromosome from degradation (11). Oxidative stress-induced reactive oxygen species and chronic inflammation are major reasons for telomere shortening (12). Therefore telomeres are reported to be closely associated with metabolism disorder, such as obesity, diabetes, NAFLD, hypertension and dyslipidemia (13-16). Previous studies suggested that shorter telomere was observed in diabetes and NAFLD, similar as insulin resistance. Recently Kim et al. identified that shorter leucocyte telomeres were associated with advanced fibrosis among USA subjects (17). Considering that the association between telomere and NAFLD-related disease may be variable in different ethnicities (18,19), together with the higher prevalence of NAFLD-relevant fibrosis in diabetic patients, our study aims to investigate whether shorter leucocyte telomeres were linked to hepatic advanced fibrosis in Chinese diabetic patients.
Methods
Study population
A total of 483 participants were T2DM patients treated at Tongji Hospital (Wuhan, China) between Jan 2012 and May 2018, 41 were excluded according to exclusion criteria. Finally, 442 Han Chinese patients, ranging from 20 to 84 years old, were admitted under the following exclusion criteria: (I) over 210 gram of alcohol drinking every week for males, (II) over 140 gram of alcohol drink every week for females, (III) coexistent liver disease, (IV) treatment with systemic corticosteroids, (V) pregnancy, (VI) secondary diabetes. This study was approved by the ethics committee of Tongji Hospital. All the procedures complied with the provisions of the Declaration of Helsinki. Informed consents were obtained from all the patients.
Definition of hepatic advanced fibrosis
Hepatic advanced fibrosis was evaluated by NFS and FIB-4 score. NFS distinguishes whether patients have or have not advanced hepatic fibrosis, using the formula: NFS = −1.675 + 0.037 × age (year) + 0.094 × body mass index (BMI; kg/m2) + 1.13 × impaired fasting glycaemia or diabetes (yes =1, no =0) + 0.99 × aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio − 0.013 × platelet (×109/L) − 0.66 × albumin (g/dL). Participants were stratified into three groups by tertiles of NFS. All the subjects were classified as one of the following three groups: advanced fibrosis (NFS >0.676), indeterminateness (NFS 0.676 to −1.455) and no-advanced fibrosis (NFS <−1.455) (20). FIB-4 score evaluates the degree of fibrosis with the following formula: FIB-4 = [age (year) × AST (U/L)]/[platelet (109/L) × ALT(U/L)1/2]. Subjects were split into 3 groups, including those with non-advanced fibrosis (FIB-4 <1.30), indeterminateness (FIB-4 1.30 to 2.67), and advanced fibrosis (FIB-4 >2.67) (21).
Measurement of terminal restriction fragment length
AxyPrep Blood Genomic DNA Miniprep kit (Axygen, Corning, Inc., NY, USA) was used for Blood Genomic DNA extraction. The concentration of DNA samples was measured by Nanodrop. Genomic DNA were digested by Hinf I (R0155L, New England Bio Labs, Beverly, MA, USA) and RsaI (R0167L, New England BioLabs) at 37 °C overnight. Then, agarose gel electrophoresis was applied to separate the digested DNA at 70 volts for 18 hours. After that, denatured the gel in a pyrex container. Neutralize the gel for 30 min after drying the gel. Then the gel was transferred to a cylindrical hybridization tube. The gel was prehybridized with 10 mL prehybridization solution for 2 hours at 37 °C. A 32P-labeled telomeric probe was used to detect telomeres. The hot probe was made with 10 µL probe system (Deionized water 3.0 µL, T4PNK buffer 1.0 µL, 32P-labeled telomeric probe 5'-(CCCTAA)3-3' [(10 pmol) 1.0 µL, [γ32-P] ATP (370MB q/mL) 3.0 µL, T4PNK (8 U/µL) 2.0 µL], then incubated at 37 °C for 1 hour and T4PNK was inactivated by heating at 68 °C for 10 min. After discarding the hybridization solution, the hot probe was added to the hybridization solution and let it hybridize overnight at 37 °C. After the gel was washed, it was then exposed to a phosphor imager and scanned with a Typhoon system (Typhoon, GE Healthcare, Wisconsin, USA) separately, and the results were visualized with Image Quant software (Molecular Dynamics, Sunnyvale, CA). The weighted mean telomere length was calculated.
Statistical analysis
All data analysis was performed using SPSS (version 22.0). A value of P<0.05 was considered statistically significant. Student’s t-test was used to test the difference between means of normally distributed data. Distribution of the continuous variables was carried out by Kolmogorov-Smirnov Test. Mann-Whitney test was applied to analysis Non-normally distributed data. χ2 test was applied to examine categorical data. Pearson’s correlations and Spearman’s correlation were performed to examine the relationship between leucocyte telomere length (LTL) and other parameters. Multiple logistic regressions were performed to identify the risk factors for advanced fibrosis. The ANOVA trend analysis with polynomial contrast was used to estimate the association between hepatic fibrosis and eGFR. The accuracy of LTL as a biomarker for advanced fibrosis in T2DM patients was calculated by area under the receiver operating characteristic (ROC) curve.
Results
Characteristics of subjects
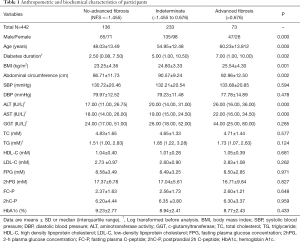
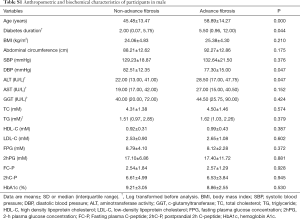
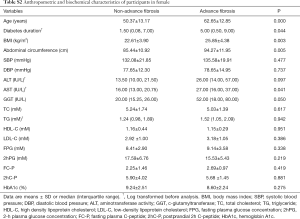
General characteristics of the subjects were summarized in Table 1. Among all the participants, 16.5% had advanced fibrosis, 30.8% had no-advanced fibrosis, and remaining 52.7% were indeterminate. T2DM patients with hepatic advanced fibrosis were older (P<0.001), and had longer diabetes duration (P=0.002). BMI, abdominal circumference, ALT and AST were also significantly higher in diabetic patients with advanced fibrosis (all P<0.005). Since telomere was related to metabolic indexes with gender difference (13), characteristics of the male and female subjects were displayed in Tables S1,S2, respectively. In addition, complications are main concerns in diabetes. We analyzed the change of diabetes-related complication in T2DM patients with different degree of hepatic fibrosis. With hepatic fibrosis worsened, renal function [assessed by glomerular filtration rate (eGFR)] also gradually decreased in our study (Figure S1A). Consistent with previous report, patients with advanced fibrosis had a higher prevalence of diabetic complications, including diabetic retinopathy and diabetic peripheral neuropathy (Figure S1B). Meanwhile, the rate of major cardiovascular events (MACE) were also increased in patients with advanced fibrosis (Figure S1C).
Full table
Full table
Full table

LTL in T2DM subjects with advance fibrosis
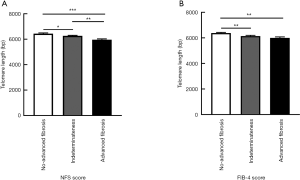
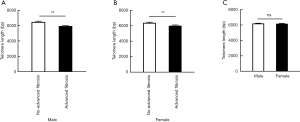
To access the association of LTL and hepatic fibrosis, we divided the participants into three groups based on their NFS or FIB-4 scores. LTL ranged from 4,909 to 8,951 bp in total. When classified by NFS score, LTL were shorter (P<0.001) along with the increased severity of hepatic fibrosis in T2DM patients. Diabetic patients with advanced fibrosis had shorter telomere length (5,959.88±62.36 bp) compared to those without advanced fibrosis (6,430.95±64.41 bp) (Figure 1A). Those with advanced fibrosis still had significant shorter LTL even after being adjusted by age (data not shown). In addition, T2DM patients with advanced fibrosis, when stratified by FIB-4 score, still had significant shorter LTL compared to those without advanced fibrosis (Figure 1B). When taking the gender into consideration, shorter LTL was observed in diabetic patients with advanced fibrosis in both male and female (Figure S2A,B). Meanwhile, LTL showed no difference between male and female (Figure S2C).
Association of LTL with age and diabetes duration
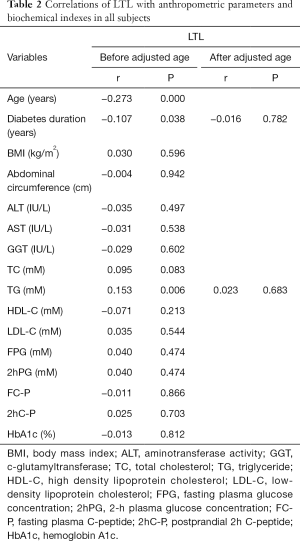
We next investigated the correlation between LTL and a cluster of anthropometric and biochemical parameters (Table 2). The analysis indicated a significant negative association of LTL with age (r=−0.273, P<0.001), diabetes duration (r=−0.107, P=0.038) and triglyceride (TG) (r=0.153, P=0.006). But the association of LTL with diabetes duration and TG was no longer significant after adjustment for age.
Full table
LTL as an independent factor of advanced fibrosis in T2DM patients
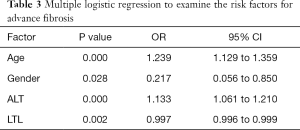
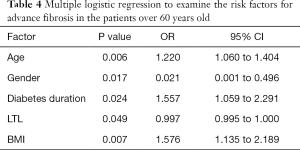
Explanatory factors of NAFLD-related advanced fibrosis in T2DM patients were evaluated by multiple logistic regressions, including gender, age, diabetes duration, BMI, abdominal circumference, ALT and AST, since these variables were significantly different between T2DM patients with and without advanced fibrosis. LTL was significantly associated with advanced fibrosis in T2DM patients (OR: 0.997, 95% CI: 0.996–0.999; P=0.002), together with age (OR: 1.239, 95% CI: 1.129–1.359; P<0.001), gender (OR: 0.217, 95% CI: 0.056–0.850; P=0.028) and ALT (OR: 1.133, 95% CI: 1.016–1.210; P<0.001) (Table 3). We also included all the parameters in Table 1 to perform logistic regressions, LTL was still significantly associated with advanced fibrosis in T2DM patients (OR: 0.996, 95% CI: 0.992–0.999; P=0.014).To further explore the effect of diabetes duration on advanced fibrosis, participants were stratified by age (20–39, 40–59, >60 years). Diabetes duration was only significantly associated with advanced fibrosis in T2DM patients over 60 years old (OR: 1.557, 95% CI: 1.059–2.291; P=0.024) (Table 4).
Full table
Full table
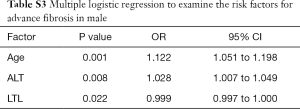
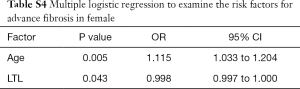
Since gender had significant effect on advanced fibrosis, we performed logistic regressions to identify independent risk factors in male and female separately. LTL was still significantly associated with advanced fibrosis in male T2DM patients (OR: 0.999, 95% CI: 0.997–1.000; P=0.022), together with age (OR: 1.122, 95% CI: 1.051–1.198; P=0.001) and with ALT (OR: 1.028, 95% CI: 1.007–1.049; P=0.008) (Table S3). Interestingly, only LTL (OR: 0.998, 95% CI: 0.997–1.000; P=0.043) and age (OR: 1.115, 95% CI: 1.033–1.204; P=0.005) were significantly related to advanced fibrosis in female T2DM patients (Table S4).
Full table
Full table
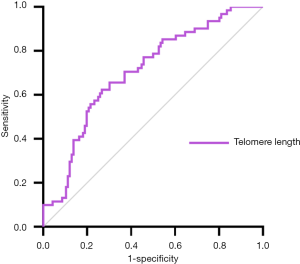
ROC curves for advanced fibrosis
Based on the above results, LTL may be a potential biomarker for NAFLD-related advanced fibrosis in T2DM patients. The ROC curve shown in Figure 2 represented the diagnostic accuracy of LTL. The area under curve was 0.707 (95% CI: 0.628–0.785). Setting cut-off value at 6,028.50bp as calculated by Youden index, sensitivity of LTL as biomarker for NAFLD-related advanced fibrosis in T2DM patients was estimated to be 62.3% and specificity to be 73.3%.
Discussion
This present cross-sectional study indicated that T2DM patients with advanced fibrosis had significantly shorter LTL in Chinese population, and LTL was negatively related to age and diabetes duration. LTL was significantly associated with the advanced fibrosis status in T2DM patients. In addition, diabetes duration only had a significant association with hepatic fibrosis when T2DM patients were over 60 years old.
The severity of hepatic fibrosis was reported to reversely relate to eGFR (22). Our study also suggested that diabetic patients with advanced fibrosis had lower eGFR. Meanwhile higher prevalence rate of MACE (4) and diabetes complications were observed in advanced fibrosis in current study, which was consistent with previous studies (23). Due to the strong relation between diversified hazard and advanced fibrosis, it’s necessary to identify the biomarker for advanced fibrosis in T2DM patients.
Our finding suggested that advanced fibrosis had significantly shorter LTL in T2DM patients, no matter when advanced fibrosis was estimated by NFS and FIB-4 score system. In fact, shorter telomeres were also reported to link to other chronic liver diseases, such as alcoholic liver disease and viral hepatitis (24,25). Furthermore, patients with cirrhosis had shorter telomere than age-matched controls, and the association of short telomere with hepatic cirrhosis could be explained by the TERE gene variants which were components of telomerase (26). Recently, Kim et.al found that short LTL was reversely correlated with liver fibrosis, and this correlation was more obvious in elder subjects as well as among non-Hispanic Whites. Diabetes and obesity could not further promote the risk of advanced fibrosis in the continuous National Health and Nutrition Examination Survey (NHANES) in USA. Considering that the correlation between LTL and NAFLD-related disease is largely affected by ethnicity (18,19,27), it is still necessary to explore the relationship between LTL and liver fibrosis in Chinese diabetic patients. In accordance with previous study (17), this study identified that shorter LTL was closely related to advanced fibrosis in Chinese diabetic patients for the first time. And this correlation was confirmed, even after adjusting for age and gender.
Our observations were in line with previous finding that LTL was negatively correlated to age and diabetes duration (27). But the correlation between LTL and diabetes duration lost statistical significance after adjustment for age. No significant association between LTL and metabolic indexes were identified in our study. It’s worth noting that the interactions between short LTL and metabolic indexes were still controversial. Nordfjall et al. found that LTL was only related to BMI in women. Meanwhile, LTL was negatively associated with high density lipoprotein (HDL) and 2hPG in man (13). And Nordfjall et al. also summarized the studies which focused on the relationship between LTL and metabolic indexes (BMI, hypertension, insulin, glucose, HDL, LDL, triglycerides, and cholesterol). Surprisingly, none of the metabolic indexes mentioned above were consistently associated with LTL.
Our results also suggested that short telomere length was a significant risk factor for advanced fibrosis status in addition to age, male and ALT in T2DM patients. One recent cross-sectional study determined several risk factors associated with biopsy proven advanced fibrosis in T2DM patients (19), such as age and ALT which was in line with our study. Male was another risk factor in our study, which was in line with identified in previous study (28). Shorter telomere length was shown to be a biomarker of NAFLD-related advance fibrosis, which was independent of age, ALT and gender. Insulin resistance causes hyperinsulinemia (29) and inflammatory cytokines (30), which may lead to liver fibrosis and hepatocellular carcinoma by promoting proliferation and reducing apoptosis within the liver. In our study, even though diabetic patients with advanced fibrosis had more serious insulin resistance (estimated by HOMA-IR) than those without advanced fibrosis (0.87±0.07 versus 0.99±0.10; P=0.537), but the difference was not significant. And logistic regressions also suggested that HOMA-IR (OR: 1.012, 95% CI: 0.987–1.038; P=0.357) was not an independent risk factor for advanced fibrosis in diabetic patients. The explanation may be that subjects in our study were diabetic patients who had severe insulin resistance, and the difference between the diabetic patients with and without advanced fibrosis is not obvious.
The specific mechanism between short telomere and fibrosis was still remains obscure so far. Telomeres are regarded to protect chromosomes tips from degradation, end-to-end fusion and compensate for the DNA loss (31). Telomeres become shorter gradually during each cell division by DNA replication, so senescence or apoptosis may trigger cellular signaling cascades which may lead to shortened telomeres (32). Chronic liver injury induced by NAFLD lead to a series of pathophysiological changes, which include hepatic regeneration, increased cellular turnover and dysfunctional telomere repairs induced progressive telomere shortening (33,34). These pathophysiological changes may finally cause liver fibrosis. And oxidative stress is supposed to lead to reactive oxygen species and chronic inflammation, which may induce telomere shortening. Based on cross-sectional and prospective studies (35,36), chronic hepatic injury was reported to be significantly associated with the T2DM, In turn, T2DM leading to oxidative stress (37) and elevation of liver enzymes, induces progressive hepatic damage and regeneration. Therefore, T2DM may further damage fatty liver, and induce progressive telomere shortening.
Longer diabetes duration was reported to have a link with liver stiffness measurement (38), and our previous study identified that the shortening rate of LTL in T2DM patients with NAFLD was higher than those without NAFLD with the extension of diabetes. But the significant association of diabetes duration with hepatic fibrosis was not observed in our study population. In order to deeply estimate the role of diabetes duration in advanced fibrosis, diabetic patients were stratified by age. Interestingly, diabetes duration as a risk factor for advanced fibrosis was identified only in T2DM patients over 60 years old. One possible explanation for no correlation between diabetes duration and advanced fibrosis in other age-subgroups possibly was due to that liver fibrosis is more obvious in the older in the older population.
The strengths and limitations were discussed as follow. First, our study uncovered that shorter LTL was associated with NAFLD-related advanced fibrosis in T2DM patients. Therefore, it may provide a clinically safe, feasible and effective method to detect advanced fibrosis by measurement of LTL. Second, the southern blot-based method, terminal restriction fragment analysis, was used in our study to measure LTL, which is considered as the gold standard. This method is more accurate and repeatable compared to quantitative PCR in other studies. There were several limitations of our study. First, our study did not apply more accurate advanced fibrosis diagnosis methods such as liver biopsy. Second, we did not apply more accurate NAFLD diagnosis methods, such as CT, MRI, or biopsy-proven steatosis. Third, our study was cross-sectional, and the sample size was relatively small. In the future, we plan to perform longitudinal studies, which will be essential to evaluate the role of telomere length as a potential predictor to assess the pathogenesis of advanced fibrosis in T2DM patients.
Conclusions
In summary, our findings may have established a critical role for short telomere length as a biomarker for NAFLD-related fibrosis in diabetic patients. In addition, our results suggest that diabetes duration can be considered as a risk factor in the detection of advanced fibrosis in old T2DM patients. Further studies will be conducted to estimate the role of short telomere length in diagnosing NAFLD-advanced fibrosis identified by liver biopsy in T2DM patients.
Acknowledgments
We would like to thank Professor Yong Zhao from Sun Yat-sen University for his technical advice and assistance.
Funding: This work was supported by the National Natural Science Foundation of China (81670754, 81600661 and 81974114).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of Tongji Hospital. Informed consents were obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896-904. [Crossref] [PubMed]
- Brunt EM, Kleiner DE. Challenges in the hepatic histopathology in non-alcoholic fatty liver disease. Gut 2017;66:1539-40. [Crossref] [PubMed]
- Younossi Z, Henry L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology 2016;150:1778-85. [Crossref] [PubMed]
- Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138-53. [Crossref] [PubMed]
- Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357-65. [Crossref] [PubMed]
- Gupte P, Amarapurkar D, Agal S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol 2004;19:854-8. [Crossref] [PubMed]
- Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 2008;28:339-50. [Crossref] [PubMed]
- Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol 2012;9:372-81. [Crossref] [PubMed]
- Mohamad B, Shah V, Onyshchenko M, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int 2016;10:632-9. [Crossref] [PubMed]
- Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018;61:1249-60. [Crossref] [PubMed]
- Mu J, Wei LX. Telomere and telomerase in oncology. Cell Res 2002;12:1-7. [Crossref] [PubMed]
- Sampson MJ, Winterbone MS, Hughes JC, et al. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006;29:283-9. [Crossref] [PubMed]
- Nordfjall K, Eliasson M, Stegmayr B, et al. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682-9. [Crossref] [PubMed]
- Revesz D, Verhoeven JE, Picard M, et al. Associations Between Cellular Aging Markers and Metabolic Syndrome: Findings From the CARDIA Study. J Clin Endocrinol Metab 2018;103:148-57. [Crossref] [PubMed]
- Tamura Y, Takubo K, Aida J, et al. Telomere attrition and diabetes mellitus. Geriatr Gerontol Int 2016;16:66-74. [Crossref] [PubMed]
- Verhulst S, Dalgard C, Labat C, et al. A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia 2016;59:1258-65. [Crossref] [PubMed]
- Kim D, Li AA, Ahmed A. Leucocyte telomere shortening is associated with nonalcoholic fatty liver disease-related advanced fibrosis. Liver Int 2018;38:1839-48. [Crossref] [PubMed]
- Wojcicki JM, Rehkopf D, Epel E, et al. Shorter Leukocyte Telomere Length in Relation to Presumed Nonalcoholic Fatty Liver Disease in Mexican-American Men in NHANES 1999-2002. Int J Hepatol 2017;2017:8435178.
- Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care 2015;38:1347-55. [Crossref] [PubMed]
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54. [Crossref] [PubMed]
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104-12. [Crossref] [PubMed]
- Targher G, Bertolini L, Rodella S, et al. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 2010;5:2166-71. [Crossref] [PubMed]
- Testa R, Olivieri F, Sirolla C, et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med 2011;28:1388-94. [Crossref] [PubMed]
- Wiemann SU, Satyanarayana A, Tsahuridu M, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. Faseb j 2002;16:935-42. [Crossref] [PubMed]
- Barnard A, Moch A, Saab S. Relationship between Telomere Maintenance and Liver Disease. Gut Liver 2019;13:11-5. [Crossref] [PubMed]
- Calado RT, Brudno J, Mehta P, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology 2011;53:1600-7. [Crossref] [PubMed]
- Gurung RL. Ethnic disparities in relationships of obesity indices with telomere length in Asians with type 2 diabetes. J Diabetes 2019;11:386-93. [Crossref] [PubMed]
- Zelber-Sagi S, Shoham D, Zvibel I, et al. Predictors for advanced fibrosis in morbidly obese non-alcoholic fatty liver patients. World J Hepatol 2017;9:91-8. [Crossref] [PubMed]
- Vigneri R, Goldfine ID, Frittitta L. Insulin, insulin receptors, and cancer. J Endocrinol Invest 2016;39:1365-76. [Crossref] [PubMed]
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012;56:704-13. [Crossref] [PubMed]
- Blackburn EH. Structure and function of telomeres. Nature 1991;350:569-73. [Crossref] [PubMed]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 2005;6:611-22. [Crossref] [PubMed]
- Donati B, Valenti L. Telomeres, NAFLD and Chronic Liver Disease. Int J Mol Sci 2016;17:383. [Crossref] [PubMed]
- Carulli L, Anzivino C. Telomere and telomerase in chronic liver disease and hepatocarcinoma. World J Gastroenterol 2014;20:6287-92. [Crossref] [PubMed]
- Kaneko K, Yatsuya H. Association of gamma-glutamyl transferase and alanine aminotransferase with type 2 diabetes mellitus incidence in middle-aged Japanese men: 12-year follow up. J Diabetes Investig 2019;10:837-45. [Crossref] [PubMed]
- Wang YL, Koh WP, Yuan JM, et al. Association between liver enzymes and incident type 2 diabetes in Singapore Chinese men and women. BMJ Open Diabetes Res Care 2016;4:e000296. [Crossref] [PubMed]
- Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015;6:456-80. [Crossref] [PubMed]
- Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359-68. [Crossref] [PubMed]