Low psoas muscle index associates with long-term mortality in cirrhosis: construction of a nomogram
Introduction
Despite accumulating evidence has unveiled the prognostic value of muscle atrophy (hereafter referred to as sarcopenia) in cirrhotics, the feasibility and generalizability of current indices remain suboptimal. As a matter of fact, a variety of clinical features, functional metrics and quantitative estimations have been employed for identifying liver cirrhosis (LC) with sarcopenia (1). Computed tomography (CT) can differentiate muscle from other body compositions, and this modality is relatively cheap, quick, readily available and highly accurate. Additionally, the skeletal muscle cross-sectional imaging on CT is not interrupted by obesity or fluid overload, common conditions in decompensated cirrhosis, thus may confer this to a potential tool for diagnosing sarcopenia (2). However, there is highly heterogeneous in the literature regarding definition of sarcopenia and avenues for diagnosis. Recently, skeletal muscle index (SMI) has been considered to be of more accuracy in determining sarcopenia and is recommended as the method of choice. However, numerous cutoff values have been extracted from different study population, which may hinder the applicability of this parameter (3-5). Moreover, the determination of SMI is always depending on specific software which is not exclusively designed for clinicians. Hamaguchi et al. showed that psoas muscle index (PMI), manually traced using CT imaging at the third lumbar vertebra level (L3), strongly correlated with SMI in healthy donor (6). On the contrary, a recent study reported low PMI inadequately recognized patients with increased risk of mortality, thus PMI could not substitute SMI (7). The discordance of prognostic impact of PMI may result from variances in lifestyle, diverse ethnicity as well as body size between the population in western and eastern countries. Collectively, the efficacy and usefulness of PMI in clinical setting is warranted for further investigation. Our group also concentrate on this crucial issue, taking into consideration PMI can be regularly retrieved within a general hospital as an easily accessible estimation.
Nomogram is broadly applied as a statistical model for prognostication in the medical field. It gives rise to an individual probability of death or tumor recurrence, by estimating relative risk score to every risk factor based on its contribution to the prognosis (8). A clinically translatable nomogram may assist physicians in treatment allocation and construction of individual follow-up trajectory. In this study, we sought to determine the association between PMI and long-term mortality in LC. Furthermore, we demonstrated sex-specific nomogram models combining PMI with conventional score to predict mortality of cirrhotics. Finally, we provided the evidence whether PMI was a robust indicator of deteriorated muscle function by analyzing the correlation between PMI and gait speed.
Methods
Study population
Patients aged over 18 years were consecutively recruited from Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital between January 2014 and June 2016. The diagnosis of LC was based on clinical, laboratory, imaging examinations, transient elastography results or biopsy confirmations. Exclusion criteria were as follows: (I) without CT scan within 3 months on index hospitalization; (II) primary liver carcinoma or other malignant tumors with or without metastasis; (III) concurrent pregnancy; (IV) lost to follow-up; (V) liver transplantation. Three hundred seventy-four LC subjects were included at initial assessment, 72, 30, 19 and 2 were excluded due to unavailable CT results upon admission, malignancies, lost to follow-up and liver transplantation, respectively. Finally, a total of 251 LC patients were left for final analysis. Informed consent was obtained from each patient. The patients were followed-up by two experienced physician in our department (X Xu and X Fan). The survival status within study period were obtained through outpatient or telephone interviews from the patients or their relatives. The current study was conducted in accordance with the Declaration of Helsinki and was approved by Ethics Committee of Tianjin Medical University General Hospital.
Assessment of clinical and laboratory parameters
We collected demographic information, clinical characteristics and laboratory data, including age, sex, body mass index (BMI), etiology of LC, presence of acute decompensated complications, platelet, hepatic function tests, coagulation examinations, serum sodium and creatinine for each enrollment in detail. The primary outcome of interest was defined as deceased of 3-year follow-up duration.
Assessment of computed tomography imaging
A spectral CT scanner (Discovery 750 HD 64-row, GE, USA) was used to achieve all CT imaging in LC patients. Two observers (L Lin and B Cui) read and analyzed the CT images. An intraobserver coefficient of variation was demonstrated to be approximately 2%. The final results were confirmed by a radiologist (H Wu) who has expertise in musculoskeletal anatomy according to tissue-specific Hounsfield unit thresholds. All collaborators were blinded to clinical parameters and patient outcomes. The PMI calculation method was done following previous report (6). In brief, we manually traced CT imaging at L3 for determining the cross-sectional areas of the right and left psoas muscle, and subsequently normalized to the squared patient height (cm2/m2) to obtain PMI value.
Assessment of gait speed
We detected self-selected (usual pace) gait speed over a 5-meter (m) marked distance from a fixed start in our wards for LC patients who could ambulate independently. The patients with severe hepatic encephalopathy (HE, as recognized by the time to complete a Numbers Connection Test of >120 s) were excluded. A stopwatch was used to record the time spent. The average of two consecutive trials were calculated and illustrated in m/s for final analysis (9). We select gait speed on account of its ease and validity across a variety of studies in multiple population which is indicative of health situations and physical performance decline (10,11). This parameter was measured by well-trained nurses. Prior to study initiation, two nurses simultaneously tested gait speed in a sample of 10 subjects in order to identify intra-observer agreement for the metrics as described by Bland and Altman (12).
X-tile analysis
The X-tile plots (Yale University School of medicine, New Haven, USA) can provide a single, global assessment of every possible way of dividing a cohort into low- and high-level marker expression. Moreover, this project gives rise to a rigorously statistical estimation by dividing a single cohort into training and validation subsets for best P value evaluation when separate training and validation cohorts are not available.
Statistical analysis
Data was demonstrated as mean ± standard deviation (SD) or median (interquartile range, IQR) as appropriate. Continuous data were compared using an independent Student t-test or the Mann-Whitney test for groups without normal distribution. Categorical variables were compared by χ2 test or Fisher's exact test. Correlations were evaluated by the Pearson correlation coefficient (r). Potential risk factors significant in univariate analysis (P<0.1) were entered into multivariate Cox proportional hazard model. To avoid redundancy, the individual components of the MELD score or CTP classification significantly associated with mortality at univariate analysis were not included in the multiple model due to the two scores already had been detected in multivariate analysis. The survival rates were calculated using the Kaplan-Meier method and compared to explore statistically significant differences using the log-rank test. The receiver operating characteristics curve (ROC) was used to evaluate sensitivity, specificity and the area under ROC (AUC). Additionally, subgroup analysis to compare the survival rate between patients with PMI stratified according to age and MELD score were performed with the Cox regression model. We considered P<0.05 as statistically significant. All statistical analyses were carried out by using Stata 14.0 (Stata Corporation, College Station, Texas, USA) and MedCalc 15.2.2 (Mariakerke, Belgium).
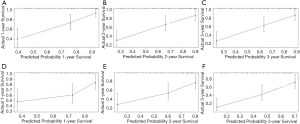
A nomogram based on the results of previous multiple analysis was established by using R version 3.3.2 (http://www.r-project.org/). Notably, all parameters were transferred to the established model as continuous value rather than arbitrary dichotomy, in order to retain more predictive efficacy. Harrell's concordance index (C-index) was used to measure the performance of the nomogram, the larger the C-index, the more accurate was the prognostic capability of proposed nomogram. The calibration curve was used to compare the agreement between nomogram and ideal observation in the study cohort. The x-axis represents the predictive survival resulting from the nomogram, and the y-axis exhibits actual survival assessed by the Kaplan-Meier method. The decision curve analysis was conducted to assess the clinical utility of the predictive nomogram by quantifying the net benefits at different threshold probabilities. The packages of rms, Hmisc were involved in this process.
Results
Patient characteristics
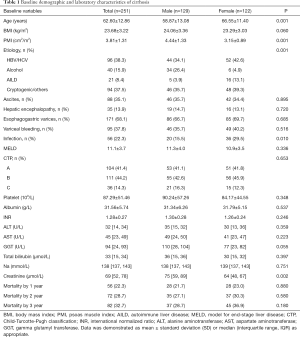
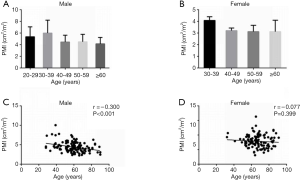
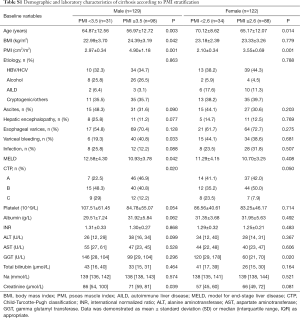
The baseline characteristics of study population are shown in Table 1. Two-hundred fifty-one consecutive LC patients were enrolled for final analysis, who met the inclusion and exclusion criteria. There were 129 males (51.4%) and mean age of the study population was 62.60±12.86 years. The etiology of LC was attributed to HBV/HCV infection in 96 (38.3%), alcohol in 40 (15.9%), autoimmune liver disease in 21 (8.4%), and cryptogenic/others in 94 (37.5%). The decompensated complications during hospitalization included ascites in 88, HE in 35, esophagogastric varices in 171, variceal bleeding in 95 and infection in 56 patients, respectively. The causes of death were attributed to liver failure in 20, severe infection in 16, cardiovascular diseases in 9, esophagogastric variceal bleeding in 13, hepatorenal syndrome in 12, HE in 7 and other reasons in 5 patients. The mean MELD score on admission was 11.1±3.7 points. The recruited population were categorized according to CTP A/B/C in 104/111/36 subjects, respectively. Mean BMI was 23.68±3.22 kg/m2 in the entire cohort. Overall mean PMI was 3.81±1.31 cm2/m2, and PMI was significantly higher in men (4.44±1.33 vs. 3.15±0.89; P<0.001). Histograms of the PMI classified in terms of age decade and gender unveiled that PMI gradually decreased with age, and a significantly inverse relationship was observed between the PMI and age in male LC patients (r =−0.300, P<0.001; Figure S1). However, we found no obvious correlation between age and PMI in female cohort.
Full table
Independent predictors for 3-year mortality in liver cirrhosis
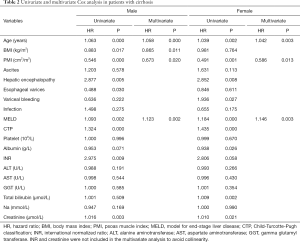
Baseline demographic, clinical and laboratory variables, including PMI for the prediction of 3-year mortality, were further investigated by univariate Cox regression model. Due to discordance with respect to PMI values, we performed sex-specific analysis. Results from the univariate analysis were as follows: age, BMI, PMI, HE, esophagogastric varices, MELD score, CTP classification, INR and creatinine were prognostic factors in males; age, PMI, HE, variceal bleeding, MELD score, CTP classification, albumin, total bilirubin and creatinine in females. After adjusting the confounders, we found that age, BMI, PMI and MELD score were independent prognostic factors in males; and age, PMI and MELD score were independent prognostic factors in females (Table 2).
Full table
Construction of predictive nomogram
Considering discrepancies in risk factors for 3-year mortality between male and female patients with LC, two prognostic nomograms by sex were retrieved based on multivariate Cox regression model in terms of the significantly independent predictors (Figure 1). A higher score calculated from the sum of the assigned value of points for every prognostic indicator in the nomogram correspond to a higher likelihood of death. For example, a male LC patient aged 65 years (62 points), with MELD of 16 (32 points), PMI of 5 (65 points) and BMI of 26 (47.5 points) would score a total of 206.5 points (and therefore have a 70%, 60% and 55% predicted risk of survival on 1-, 2-, and 3-year follow-up).

Performance of executed model and clinical usefulness
The discriminative ability of our final model was evaluated using the C-index. The nomogram of male LC for 3-year mortality had C-index of 0.792 (95% CI: 0.723–0.861), which was superior to that of MELD (0.671, 95% CI: 0.582–0.760; P<0.01) and CTP (0.671, 95% CI: 0.585–0.757; P<0.01). Similarly, the C-index for female was 0.715 (95% CI: 0.637–0.793) of the nomogram, which performed a better predictive power than MELD (0.612, 95% CI: 0.522–0.702; P<0.05) and CTP (0.650, 95% CI: 0.568–0.732; P<0.05). Figure 2 demonstrated the calibration curve of the nomogram. The calibration plots unraveled satisfactory accordance between nomogram prediction and actual observation for probability of 1-year (Figure 2A,D), 2-year (Figure 2B,E) and 3-year (Figure 2C,F) survival. The decision curve analysis indicated that the net benefits added from the application of our nomogram at 1-year, 2-year and 3-year as threshold probabilities were 0.05, 0.09, and 0.1 in males, and 0.15, 0.15 and 0.16 in females, respectively (Figure 3).

Identification of PMI value for specific follow-up trajectory
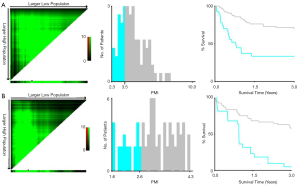
A major concern about the applicability of PMI, as well as SMI, is highly heterogeneous in terms of cutoffs for determination of sarcopenia in the medical literature. Numerous thresholds have been proposed potentially due in part to different study population (health donor vs. LC subjects), selective statistical approach (mean – 2SD vs. highest Youden’s index) along with distinct geographical location (East vs. West). From the point of view of clinician, we are predominantly concerned with whether the obtained PMI threshold could effectively differentiate LC into low or high risk of mortality, which being beneficial for conceiving specific follow-up trajectory. Collectively, X-tile project was employed to detect optimal cutpoint of PMI in terms of 3-year patient decease in the entire cohort. Consequently, the threshold of 3.5 cm2/m2 in males and of 2.6 cm2/m2 in females enabled to most significantly identify the poor and favorable outcomes in cirrhotics (Figure S2, Table S1).
Full table
The prognostic significance of PMI for 3-year mortality
While employing abovementioned cutoffs to stratify patients into two categories by sex, it also represented significant differences between Kaplan-Meier survival curves (Figure 4). Male patients with PMI ≥3.5 had significantly higher 3-year survival rate than patients with PMI <3.5 (69.3% vs. 35.7%, P=0.0002). Similarly, female patients with PMI <2.6 exhibited significantly lower 3-year survival rate than patients with PMI ≥2.6 (4.5% vs. 57.8%, P<0.0001). Furthermore, the AUCs for PMI, MELD and CTP were 0.755, 0.708 and 0.753 in male, and 0.616, 0.582 and 0.590 in female, respectively.

Prognostic impact of PMI according to the category of age and model for end-stage liver disease
We further compared the prognosis between these groups by using subgroup analysis (Figure S3). In a subgroup analysis using age, PMI was associated with increased mortality in both male and female patients. The probability of decease was significantly higher in patients with low-PMI regardless of age. In particular, the survival of patients aged less than 65 years with low-PMI was similar to that of patients aged not less than 65 years with high-PMI (Figure 5A).

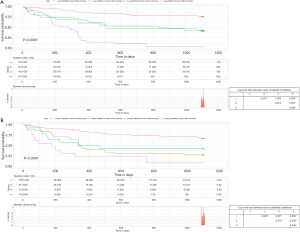
In a subgroup analysis by MELD score, PMI was an independent predictor of mortality in patients with MELD <15 (male: HR =0.60, 95% CI: 0.42–0.86, P=0.005; female: HR =0.43, 95% CI: 0.26–0.69, P=0.001). In contrast, PMI was not favorable prognostic factor in LC patients with MELD ≥15. Of note, the survival of the low-MELD score group (MELD <15) with high-PMI was not significantly different from that of the high-MELD score group with low-PMI (Figure 5B).
Association between PMI and 5-meter gait speed
At our discretion, the paramount issue is whether low PMI truly reflect muscle dysfunction or physical inability. Thus we evaluated the association between PMI and 5-m gait speed. First, we assessed reproducibility of gait speed measurements by our well-trained nurses in a separate study in which two different observers measured gait speed on 2 different repetitions of the tests by 10 participants. As graphically shown by Bland-Altman plots, there was minimal variation of the average gait speeds among the two raters (Figure S4).
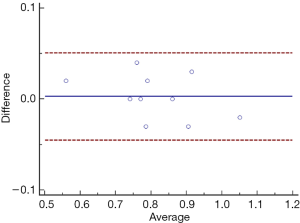
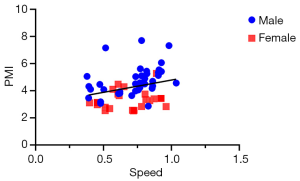
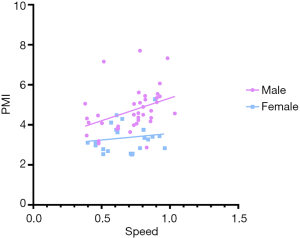
Pearson correlation coefficients were implemented to analyze the relationship between PMI and 5-m gait speed in sixty-three patients. A statistically significant correlation was found between PMI and 5-m gait speed (r =0.324, P=0.010, Figure 6). We also addressed a significant correlation between PMI and gait speed in male cirrhotics, while this correlation was not consistent in female subjects (Figure S5).
Discussion
In the present study, we evaluated the prognostic impact of PMI, a readily attainable measure on CT imaging, to predict long-term survival in our cohort of LC patients. We found that distinct predictors were independently associated with 3-year all-cause mortality by sex stratification, thereby achieved two prognostic nomogram models. Our nomogram assessment resulted in an accuracy of 0.792 in males and 0.715 in females, respectively. Moreover, we identified a moderately positive correlation between gait speed and PMI in male cirrhotics for the first time.
Over the past decade, muscular body composition is gaining increasing attention as determinants of outcomes in LC. Sarcopenia, defined as loss of muscle strength and muscle mass, has been proven to be associated with poor prognosis in decompensated cirrhotics (13,14). Notably, a major obstacle, which hampers the generalizability and feasibility, is divergent definition of sarcopenia in the literature (15). Each study done previously on sarcopenia has used its own definition, and there is no unanimity in terms of applicable cutoff for diagnosing sarcopenia, validated tools for assessment, and prognostication of explicit outcome. From the point of view of clinicians, we therefore sought to establish a fairly ideal model by maintaining the prognostic value of sarcopenia to the greatest extent. In addition, we also consider the convenience of modalities that can be performed within a general hospital using easily accessible metrics. As a result, we obtained sex-specific nomogram models incorporating PMI and other clinical parameters.
Nomogram provides information tailored to the individuals, by demonstrating a simple graphical representation of a statistical predictive model which generates a numerical probability of a clinical event (16). In the present study, we first determined independent predictors for mortality in LC patients by gender, taking into consideration the importance and necessity of sex classification in cirrhosis associated mortality studies (17). Intriguingly, we retrieved a 2-compartment prediction model based on obtained indices. Specifically, our male cirrhosis predictive nomogram include age, MELD, PMI and BMI; while age, MELD and PMI were determined as precipitating factors for females. We speculate above findings partly reflect pathophysiologic significance of major body compositions underpinning crosstalk between fat deposit and muscle atrophy driving the progression of cirrhosis and complications (18).
PMI was originally proposed by Hamaguchi et al. in a Japan cohort of adult donors for living donor liver transplantation (6). A previous study conducted in North America implied that low PMI identified an incomplete subset of patients at increased risk of mortality stratified by SMI (7). However, another western group explored the impact of malnutrition and sarcopenia (patients in the lowest sex-stratified L3-PMI quartiles were deemed as sarcopenic) on mortality and morbidity in post-liver transplant cirrhotic subjects, in a distinct population from ours, showing low PMI was independent predictor of hospital stay >20 days, 1-year mortality, and relevant to higher ICU stay/incidence of infections (19). Besides confirming that low PMI is a predictor of long-term mortality in LC, our subgroup analysis also suggested that the relationship between low PMI and poor outcomes are dependent on different stage of liver function (7). In patients with MELD >15, other precipitating factors, such as nutritional status or cirrhosis-associated immune dysfunction, should be always taken into consideration for risk stratification (19,20). In particular, the cutpoints of PMI based on our research is relatively lower than that of previous reports (7,21,22). We could interpret the reasons as follows: (I) The recruited study population included a higher proportion of LC patients with advanced age (43.4% participants aged over 65). Kim et al. indicated that cutoff values of low skeletal muscle mass dramatically decreased as age increased, and thresholds of PMI were 3.74 cm2/m2 for men and 2.20 cm2/m2 for women aged over sixty in healthy adults (23); (II) Current study aimed at differentiating enrolled subjects into distinct outcomes rather than establishment of new diagnostic criteria; (III) Our statistical consideration resulted in selection of X-tile package.
Our results also indicated that PMI had similar diagnostic accuracy in comparison with MELD and CTP. Recently, a comprehensive systemic review concluded that both MELD and CTP scores had comparable prognostic values in most of cases, although their benefit might be heterogeneous in some circumstances (24). However, deteriorations in homeostasis between liver and muscular tissue may exacerbate disease process and lead to occurrence of cirrhosis-related complications, which can not be fully captured by MELD/CTP classification. What makes sarcopenia such a unique risk factor for LC is that, unlike more traditional predictors such as age, sex or MELD, muscle depletion is potentially modifiable with exercise and nutritional intervention (25,26). For instance, Kitajima and colleagues showed that dietary supplementation with branched-chain amino acid was associated with maintenance of skeletal muscle mass (27). Collectively, we proposed that PMI may be a valid and feasible indicator of sarcopenia in LC.
Unsurprisingly, we confirmed a clear sex difference regarding the prognostic meaning of PMI. The C-statistic of retrieved nomogram was higher in male LC in comparison with that in females (0.792 vs. 0.715). Meanwhile, the decreased PMI was not closely correlated with diminished gait speed, an indicator of muscle dysfunction, in female patients. As a matter of fact, several previous studies have emphasized the sex-specific parameters while predicting various outcomes in LC. For instance, Rodrigues et al. showed that fat loss was related to portal hypertension in females other than males (28). Furthermore, a recent study implicated that subcutaneous adipose tissue index in females and SMI in males were significant predictors of overall mortality in cirrhotics (17). We assume that decreased PMI may imply distinct role contributing to the progression of cirrhosis and onset of decompensated events by sex. The inverse correlation between gait speed and PMI in males may predominantly reveal physical inactivity and protein degradation, while inflammatory crosstalk between fat, muscle and liver co-existing in female LC patients should be taken into consideration for further studies (15).
It has been realized that higher BMI is related to survival benefit in patients with a wide range of entities, including critical illness, septic insults, post-trauma events as well as malignancies (29). Further studies also found elevated BMI was associated with lower mortality in cirrhosis (30,31). Our results confirmed the strength of abovementioned correlation of male mortality in cirrhosis, while this association has not been consistent in female patients. Actually, considerable difference does exist relevant to body composition between the genders, with males encompassing more muscularity and females having more adiposity. It seems that BMI may not be indicative of adipose tissue and skeletal muscle, both of which represent diverse features and functions (5). Notably, fat loss, storage energy exhaustion along with malnourished status may serve as pivotal indicator for worsening outcome in females with cirrhosis (28,32). However, the predictive impact of BMI should be interpreted with caution since some evidence suggest that BMI is positively associated with worse mortality or cardiovascular risk in non-alcoholic fatty liver disease (33-35).
Gait speed is regarded as a highly reliable test for sarcopenia with ease of use. It strongly implies physical performance of whole-body function relevant to locomotion (36). Additionally, it is suggested that a single cutoff speed ≤0.8 m/s indicates severe sarcopenia on 4-m usual walking speed test. Moreover, recent study addressed that gait speed remained an independent and potentially modifiable risk factor for cirrhosis morbidities requiring hospitalization while grip strength did not (9). Taken into account of hard end-points we have been concerned about (3-year mortality), a positive relationship between PMI and 5-m gait speed may confer added value to our findings.
Several unique features of the present study should be addressed. First, this the first study aiming to develop a nomogram with indicator of muscle depletion on CT imaging. Of note, we confer the value of PMI to continuous risk score on nomogram scale, rather than transfer it to arbitrary dichotomization as previously reported. We assume this statistical maneuver is state-of-the-art, taking account of the integrity of data. Second, we performed subgroup analysis and showed that the impact of PMI might be less pronounced in patients with advanced MELD score, which is in keeping with previous report in terms of SMI (3). The applicable population of muscle mass index should be clarified in future. In addition, current study showed an association between PMI and one of the components of muscle characteristics in sarcopenic subjects: gait speed. This potentially complement most previous retrospective studies to date only relying on the assessment of muscle mass loss (myopenia) (37). As a matter of fact, the diagnostic algorithm of severe sarcopenia should encompass a combination of low muscle strength, low muscle quantity and quality along with poor physical performance (36).
Our study still has several limitations. First, this study was performed retrospectively in nature. Second, we were unable to validate the proposed nomogram models internally or externally. We agreed that our findings may not be generalized to other regions and populations; however, this is an ongoing longitudinal research with active enrollment of cirrhotics. Third, the approach for driving cutpoint of low PMI was totally different in our study. We utilized X-tile package, which has been widely adopted in field of biomedicine, to determine optimal PMI threshold (38-40). We regard this a deliberate choice, as the purpose of present study is to categorize cirrhotics of whom should be treated with close monitoring strategy and intensive follow-up trajectory. Lastly, from the entire cohort, gait speed profile were only available in sixty-three cases.
In conclusion, we proposed a refined nomogram incorporating PMI which rendered an individualized predictive tool for long-term mortality in LC. Moreover, PMI may be an available indicator of impaired physical dysfunction closely related to cirrhosis associated mortality. Additional validation in larger cohorts of LC patients is necessary to corroborate our findings prior to widespread transferability of this novel model for prognostication.
Acknowledgments
We thank all nurses taking part in our research.
Funding: This work was partly supported by Youth Fund of TJMUGH (ZYYFY 2015007) and the National Natural Science Foundation of China (81900487) to X.X. The funding organizations did not participate in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Footnote
Conflicts of Interest: CS serves as an unpaid section editor of Annals of Translational Medicine from Oct 2019 to Sep 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from each patient. The current study was conducted in accordance with the Declaration of Helsinki and was approved by Ethics Committee of Tianjin Medical University General Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sinclair M, Gow PJ, Grossmann M, et al. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther 2016;43:765-77. [Crossref] [PubMed]
- Heymsfield SB. Development of imaging methods to assess adiposity and metabolism. Int J Obes (Lond) 2008;32 Suppl 7:S76-82. [Crossref] [PubMed]
- Kang SH, Jeong WK, Baik SK, et al. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018;9:860-70. [Crossref] [PubMed]
- Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625-33. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200-5. [Crossref] [PubMed]
- Ebadi M, Wang CW, Lai JC, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053-62. [Crossref] [PubMed]
- Jiang X, Su Z, Wang Y, et al. Prognostic nomogram for acute pancreatitis patients: An analysis of publicly electronic healthcare records in intensive care unit. J Crit Care 2019;50:213-20. [Crossref] [PubMed]
- Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am J Gastroenterol 2016;111:1768-75. [Crossref] [PubMed]
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50-8. [Crossref] [PubMed]
- Pamoukdjian F, Paillaud E, Zelek L, et al. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J Geriatr Oncol 2015;6:484-96. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int 2011;31:1250-8. [Crossref] [PubMed]
- Durand F, Buyse S, Francoz C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014;60:1151-7. [Crossref] [PubMed]
- Ooi PH, Hager A, Mazurak VC, et al. Sarcopenia in Chronic Liver Disease: Impact on Outcomes. Liver Transpl 2019;25:1422-38. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Ebadi M, Tandon P, Moctezuma-Velazquez C, et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 2018;69:608-16. [Crossref] [PubMed]
- Mehta G, Gustot T, Mookerjee RP, et al. Inflammation and portal hypertension - the undiscovered country. J Hepatol 2014;61:155-63. [Crossref] [PubMed]
- Kalafateli M, Mantzoukis K, Choi Yau Y, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle 2017;8:113-21. [Crossref] [PubMed]
- Lin L, Yang F, Wang Y, et al. Prognostic nomogram incorporating neutrophil-to-lymphocyte ratio for early mortality in decompensated liver cirrhosis. Int Immunopharmacol 2018;56:58-64. [Crossref] [PubMed]
- Shirai H, Kaido T, Hamaguchi Y, et al. Preoperative low muscle mass has a strong negative effect on pulmonary function in patients undergoing living donor liver transplantation. Nutrition 2018;45:1-10. [Crossref] [PubMed]
- Hammad A, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic overweight on the outcomes after living donor liver transplantation. Hepatobiliary Surg Nutr 2017;6:367-78. [Crossref] [PubMed]
- Kim JS, Kim WY, Park HK, et al. Simple Age Specific Cutoff Value for Sarcopenia Evaluated by Computed Tomography. Ann Nutr Metab 2017;71:157-63. [Crossref] [PubMed]
- Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore) 2016;95:e2877. [Crossref] [PubMed]
- Duarte-Rojo A, Ruiz-Margain A, Montano-Loza AJ, et al. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122-39. [Crossref] [PubMed]
- Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: Translating evidence and experience to practice. J Hepatol 2018;69:1164-77. [Crossref] [PubMed]
- Kitajima Y, Takahashi H, Akiyama T, et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol 2018;53:427-37. [Crossref] [PubMed]
- Rodrigues SG, Brabandt B, Stirnimann G, et al. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int Liver Int 2019;39:1672-81. [Crossref] [PubMed]
- Cheung YM, Joham A, Marks S, et al. The obesity paradox: an endocrine perspective. Intern Med J 2017;47:727-33. [Crossref] [PubMed]
- Dick AA, Spitzer AL, Seifert CF, et al. Liver transplantation at the extremes of the body mass index. Liver Transpl 2009;15:968-77. [Crossref] [PubMed]
- Chang SH, Liu X, Carlsson NP, et al. Reexamining the Association of Body Mass Index With Overall Survival Outcomes After Liver Transplantation. Transplant Direct 2017;3:e172. [Crossref] [PubMed]
- Campillo B, Richardet JP, Scherman E, et al. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition 2003;19:515-21. [Crossref] [PubMed]
- Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608-12. [Crossref] [PubMed]
- Heuer M, Kaiser GM, Kahraman A, et al. Liver transplantation in nonalcoholic steatohepatitis is associated with high mortality and post-transplant complications: a single-center experience. Digestion 2012;86:107-13. [Crossref] [PubMed]
- Sánchez-Jiménez BA, Brizuela-Alcantara D, Ramos-Ostos MH, et al. Both alcoholic and non-alcoholic steatohepatitis association with cardiovascular risk and liver fibrosis. Alcohol 2018;69:63-7. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Rodrigues SG, Brabandt B, Stirnimann G, et al. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int 2019;39:1672-81. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Zhang W, Wang X, Jiang R, et al. Effect of Tumor Size on Cancer-Specific Survival in Small Hepatocellular Carcinoma. Mayo Clin Proc 2015;90:1187-95. [Crossref] [PubMed]
- Tan Z, Ma G, Yang H, et al. Can lymph node ratio replace pn categories in the tumor-node-metastasis classification system for esophageal cancer? J Thorac Oncol 2014;9:1214-21. [Crossref] [PubMed]