
The diagnostic value of serological tests and real-time polymerase chain reaction in children with acute Mycoplasma pneumoniae infection
Introduction
Mycoplasma pneumoniae (MP) is a prominent cause of infections of the upper and lower respiratory tract in children and has a particular association with community-acquired pneumonia (CAP) (1). In recent years, MP has been attributed with up to 40% of CAP cases in children (2-4). Based on its clinical presentation, Mycoplasma pneumoniae pneumonia (MPP) is often difficult to distinguish from pneumonia originating from other pathogens (5), and the problem has been exacerbated by the indiscriminate use of macrolides, which has resulted in the spread of drug-resistant strains of MP (6).Therefore, the prompt clinical treatment of MP infection hinges on rapid and reliable laboratory diagnosis.
Polymerase chain reaction (PCR) is a widely accepted method of diagnosis because of its speed and high sensitivity and specificity (7). Serological studies are more sensitive than culture for the detection of acute infections and have sensitivity comparable to PCR; however, they are dependent on antibodies, which require sufficient time to develop after infection and the patient to have a functional immune system (8).The immune system functions differently in children of different ages, which can lead to variations in innate and adaptive immune response and affect the expression of antibodies (9). Moreover, in some studies, the sensitivity of real-time polymerase chain reaction (RT-PCR) has been shown to decrease as the time between symptom onset and sample collection increases (9,10). Thus, an optimal detection method should be recommended for children of different ages and those with different disease durations, respectively. Therefore, we analyzed various early serological and molecular diagnostic tests to determine their clinical significance and usefulness in children of different ages infected with MP who had different disease durations. In investigating the optimal test, bronchoalveolar lavage fluid (BALF) PCR served as the gold standard (7,11,12).
Methods
Patients
The clinical information of pediatric patients with respiratory infection from January 2015 to October 2018 was collected and analyzed in this retrospective study. Patients were selected from a database which had been established for children with lower respiratory tract infections (LRTIs) in the Department of Respiratory Medicine, Children's Hospital, Soochow University.
We mainly enrolled pediatric patients under the age of 16 years who met the following criteria: (I) eligible for bronchoalveolar lavage procedures, with clinical manifestations of persistent chest radiographic infiltration and/or respiratory symptoms, such as wheezing, cough, increased dyspnea, unexplained hypoxemia; and (II) nasopharyngeal aspirates (NPA) and BALF samples were collected and analyzed by RT-PCR, and the serum titers of MP, immunoglobulin M (IgM) and immunoglobulin G (IgG),were checked in both the acute and convalescent phases. Patients with immunosuppression and those who were receiving immunosuppressive treatment were not included in the study.
A total of 296 children who met the criteria were enrolled, and 164 patients were confirmed mycoplasma infection by BALF PCR test. Detailed clinical history was recorded, and the following laboratory tests were conducted including blood routine, C-reactive-protein (CRP), lactate dehydrogenase (LDH), biochemical functions, humoral-immunity, and lymphocyte subsets. The study received approval from the Institutional Human Ethical Committee of Children’s Hospital of Soochow University (No. 2020LW002), and written consent was obtained from the parent/guardian of each child participating in this study.
Study method
Specimen collection
Venous blood samples were collected within 24 hours of admission and immediately sent to the laboratory. A second peripheral blood sample was collected from each patient before discharge. NPA samples were taken from the patients within 24 hours of admission via a sterile plastic catheter introduced through the nasal passage into the lower part of the pharynx. Bronchoscopy was performed under local anesthesia, following standard hospital procedures. Sterile normal saline was instilled into the, most severely infected area or the right middle lobe with LRTI, and then sucked back into a sterile container with a negative pressure of 3.33–13.3 kPa. The 2–3 mL NPA specimens were immediately sent together with the first BALF aliquot to the laboratory for the detection of respiratory pathogens. All NPA and BAL procedures were performed by experienced staff in accordance with standardized clinical procedures.
Serology of MP
The ELISA kit (Shenzhen YHLO Biotech, Shenzhen, China) was used to detect MP antibodies (IgM and IgG) in serum samples taken from the children in the acute phase (on admission) and convalescent phase (on discharge), in accordance with the manufacturer’s instructions.
Acute MP infection was confirmed either by a single positive serum IgM titer (cut-off 1.1 S/CO) or a 1.5-fold increase in IgG titer (cut-off 24 RU/mL) in the convalescent serum sample (13).
RT PCR for MP detection
A RT PCR procedure (DaAn Gene Co. Ltd., Guangzhou, China) was used to detect MP in real time (14) (approved by the State Food and Drug Administration of China). Briefly, both the samples of NPA and BALF were shaken for 30 s and centrifuged at 15,000 ×g for 5 min. The sediment was collected and the DNA was extracted from a 400 µL aliquot in line with the manufacturer’s instructions. PCR amplification was performed using primers and probes purchased from DaAn Gene Co. Ltd., in a 7600 RT PCR system (Applied Biosystems, Foster City, CA, USA). The PCR conditions were as follows: 93 °C for 2 min; 10 cycles of 93 °C for 45 s and 55 °C for 60 s; 30 cycles of 93 °C for 30 s and 55 °C for 45 s. Quantitative curves were drawn for standard control samples at different concentrations. In the BALF samples, MP positive was indicated by MP DNA qualitative positive. To exclude MP colonization, in the NPA samples, MP-positive samples were defined as those with DNA concentrations ≥1×104 copies/mL. In the combined IgM and NPA PCR test, MP-positive infection was indicated when either one or both of the tests were positive.
Detection of co-infection with other respiratory pathogens
The BALF and NPA specimens were screened for bacteria using culture, and the quantitative specimen cultures were considered positive when the growth of ≥105 colony forming units per ml (cfu/mL) was observed. Respiratory viruses, such as influenza virus (IFV), respiratory syncytial virus (RSV), parainfluenza virus (PIV), and adenovirus (ADV) were detected by direct immunofluorescence assays (15), while human bocavirus (HBoV) and rhino virus (HRV) were tested by reverse transcription PCR (16).
Statistical analysis
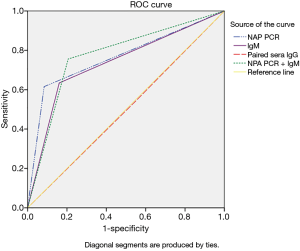
Statistical analysis was carried out using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). Normal distribution of measurement data was expressed as (mean ± standard deviation), Non-normal distribution of measurement data was expressed as median (quartile spacing), and the two groups were compared using the Wilcoxon test. The chi-square test was applied for numerical data (Table 1). One-way analysis of variance (ANOVA) was used to calculate the differences in the positive rate between different groups (Figure 1, Tables 2,3). Correlation between the four different diagnostic methods was assessed by kappa analysis, and MP infection was diagnosed by BALF PCR. The validity of the different diagnostic methods, including specificity, sensitivity, and positive and negative predictive values were calculated with reference to the BALF PCR test results. Receiver operating characteristic (ROC) curves were plotted using SPSS 20.0 software, and the areas under the curves (AUCs) were calculated to assess significant differences between the diagnostic methods. Statistical significance was indicated when P<0.05 (Figure 2).
Full table

Full table
Full table
Results
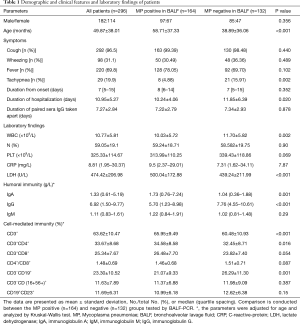
Demographic and clinical features and the laboratory findings
The 296 patients enrolled in our study, of whom 182 (61.49%) were maleand114 (38.51%) were female, had a mean age of 49.87±38.01 months. The majority of the patients had a cough (n=292, 96.5%) or fever (n=220, 69.8%). The median duration of symptom onset was 7 days, and the median time between the paired sera collection for IgG quantification was 7.27 days. Overall, 164 patients tested positive for MP infection by BALF PCR. With a median age of 58.71±37.33 months, the patients who were MP positive were younger than those who tested MP negative (38.89±36.06 months, P<0.001). On admission, most of the other symptoms were similar across both groups. The MP-positive groups had higher levels of LDH (P<0.001) and IgA (P=0.001), and a lower level of IgG (P<0.001). The MP-positive group positive also showed higher levels of CD3+ cells (P<0.001) and CD3+CD4+ cells (P=0.016), and a lower level of CD3− CD19+ cells (P<0.001) (Table 1).
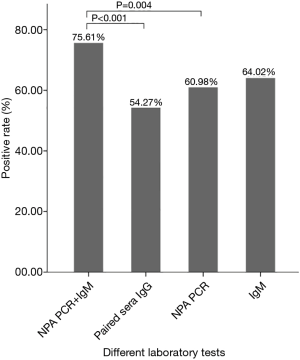
Positive results of different laboratory tests in 164 children with MP infection confirmed by BALF PCR
Among the 164 children diagnosed with MP infection by BALF PCR, 124 (75.61%) showed a positive result for combined IgM and NPA PCR, which was significantly higher than that for NPA PCR (n=100, 60.98%) and paired sera IgG (n=89, 54.27%), and 105 (64.02%) had a positive result for IgM (Figure 1).
With BALF PCR serving as the gold standard, the ROC curve was generated for each diagnostic test (Figure 2). The IgM and NPA PCR tests were shown to have the highest diagnostic accuracy, and the area under the ROC curve for NPA PCR + IgM (0.775) was higher when compared with those for IgM (0.737) (P<0.001, χ2 test)and paired sera IgG (0.503) (P<0.001, χ2 test) alone. The area under the ROC curve for NPAPCR was 0.766. The sensitivity and specificity of combined IgM and NPA PCR were 82.12% and 72.41%, respectively, and the PPV and NPV of NPA PCR + IgM were 76.61% and 79.55%, respectively.
Co-infection with MP
In the 164 children with MP infection confirmed by BALF PCR, MP was the only pathogen detected in 92 patients, while the remaining 72 (43.90%) had co-infection with other pathogens including respiratory viruses [37], HRV [12], HBoV [9], RSV [8], ADV [5], IFV-B [2], and IFV-A [1]. Bacterial infections were found in 19 patients, 2 were diagnosed with Chlamydia pneumoniae (CP), and 14 were positive for two or more additional pathogens.
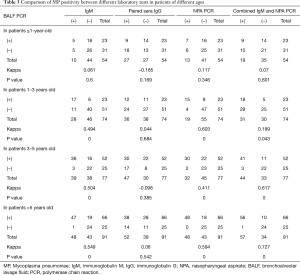
Comparison of MP positivity among different laboratory tests in children of different ages
The 164 children confirmed with MP infection by BALF PCR had a median age was 58.71±37.3 months and comprised 23 patients ≤1 year old, 23 patients aged 1–3 years old, 52 patients aged 3–5 years old, and 66 patients >5 years old. Positive results among the different age groups were compared using the same diagnostic test. The MP positive rate evaluated by IgM test in children <1 year old was 21.74%, which was lower than children aged 1–3, 3–5, and >5 years (all P<0.0083). The MP positive rate of NPA PCR test in children >5 years was 84.85%, which was higher than that of children <1 year old (P<0.0083). The MP positive rate evaluated by the combined IgM and NPA PCR test in children aged 3–5 years and those >5 years was 78.85% and 84.85%, respectively, which was higher than the results of children <1 year (P<0.0083). Comparisons were also made between the positive results of the different diagnostic tests on patients of the same age. The MP positive rate of the combined IgM and NPA PCR test in children >5 years old was 84.85%, which was higher than that of the paired sera IgG test (P<0.0083) (Table 2).
When BALF PCR was used as the gold standard, the MP detection rate of the NPA PCR test in children aged 1–3 years was consistent with that of the BALF PCR test (Kappa =0.603). The MP detection rate of the combined IgM and NPA PCR test in children aged 3–5 years old was consistent with that of BALF PCR test (Kappa =0.617). In children >5 years, the MP detection rate of the combined IgM and NPA PCR test was consistent with that of BALF PCR test (Kappa =0.727), and the detection rates of the independent NPA PCR and IgM tests were both moderately consistent with that of the BALF PCR test (Kappa =0.549, 0.594). Moreover, there was a correlation between the detection rates of the NPA PCR and IgM tests (Kappa =0.824) (Table 3).
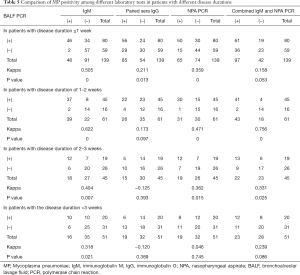
Comparison of MP positivity among different laboratory tests in children with different disease durations
Among the164 children diagnosed with MP infection by BALF PCR, the median duration of symptom onset was 7 days. Regarding disease duration, 80, 45, 19, and 20 patients had a disease duration <1, 1–2, 2–3 weeks, and >3 weeks, respectively. Comparisons were made between the different diagnostic tests for children with different disease durations. The positivity rates evaluated by IgM test in children with a disease duration of 1–2 weeks was 82.22%, which was higher than that of children with a duration <1 week and >3 weeks (all P<0.0083). The MP positive rates of the paired sera IgG test in children with a disease duration <1 week was 70%, which was higher than that children with a duration of 2–3 weeks or >3 weeks (all P<0.0083). The MP positive rates of the combined IgM and NPA PCR test in children with a disease duration <1 week or 1–2 weeks was 70% and 91.11%, respectively, which was higher than that of >3 weeks (all P<0.0083). Comparisons were made between the positive results of the different diagnostic tests in patients with the same duration from onset. The MP positive rates of the NPA MP + IgM test in children with a disease duration of 1–2 weeks was 91.11%, which was higher than that of the paired sera IgG test and NPA PCR test (all P<0.0083).The MP positive rates of the combined IgM and NPA PCR test in children with a duration of 2–3 weeks was 68.42%, which was higher than that of the paired sera IgG test (all P<0.0083) (Table 4).

Full table
When BALF PCR was used as the gold standard for diagnosis of children with a disease duration <1 week, the MP detection rate of the IgM test was moderately consistent with that of the BALF PCR test (Kappa =0.505), and the detection rates of the NPA PCR test and IgM were not consistent with each other (Kappa =0.438). The detection rate of the combined IgM and NPA PCR test in children with a disease duration of 1–2 weeks was correlated with that of the BALF PCR test (Kappa =0.756). In children with a disease duration of 2–3 weeks, none of the four diagnostic tests were consistent with that of BALF PCR test, while the detection rates of NPA PCR test and IgM were well correlated with each other (Kappa =0.771) (Table 5).
Full table
Discussion
Early diagnosis of MP infection in children remains clinically challenging due to its nonspecific clinical manifestations as well as a lack of both accurate and quick laboratory tests. Diagnosis of MP by culture is a traditional gold standard test, but it is time-consuming and cumbersome. Recent studies have suggested that applying PCR to BALF could serve as a new gold standard test for diagnosing MP infection because of its high sensitivity and specificity, and can minimize cases of colonization (7,11,12). In our study, BALF PCR test was used as the gold standard to compare different diagnostic methods for early detection of MP infection in children.
In our study, 164 patients tested positive for MP infection by BALF PCR, with a positive rate of 55.41%, which is higher than that reported in previous studies (5,17). Many of the children enrolled in this study were already suffering with lobar pneumonia, showed persistent chest radiographic infiltration, and were eligible for bronchoalveolar lavage procedures. Moreover, MP was established in our previous studies as the major pathogen of lobar pneumonia, accounting for 88% of all pathogens (date not published).
The serological assay, which relies on the pathogen-induced production of antibodies, is often used to diagnose MP infection. Various assays, such as direct and indirect hemagglutination assays that use IgM capture, immunofluorescence antibody assays, particle agglutination antibody assays, and enzyme immunoassays (EIAs) are widely available. IgM is considered to be an early indicator of MP infection, as it appears in the first week of the disease and reaches peak titers in the third week. After declining to low basal titer levels it may be present for up to several years (8); therefore, IgM detection is more reliable for diagnosing children than elderly people (18). Two recent studies have reported the positivity rate of MP-specific IgM for pediatric patients as 48.2% and 63.6%, respectively (19,20). In our study, the positivity of IgM test was 64.02%, but its value was limited for diagnosing MP infection in children under 1 year of age, for whom the positivity rate was only 21.74% compared with 71.21% for children over 5 years of age. Since the antibody response to IgM is not often induced in very young children (21). The children with a disease onset of 1–2 weeks showed the highest positivity rate (82.22%), and a detection rate consistent with BALF PCR. Previous studies by Lee et al. showed that 32.5% of patients with MP did not have a positivity IgM titer during the first several days of symptoms, and IgM only became positive after 2 weeks of follow up (19). Our results were consistent with these earlier reports.
As is commonly understood, a positive conversion or a significant increase in IgG antibody titer in paired sera may take at least 2 weeks, and can indicate an acute MP infection. However, taking two consecutive blood samples 2 weeks apart is too late to assist with guiding treatment decisions, and it is sometimes difficult to perform clinically in children (17). In our study, the median time gap of the collection of paired sera was 7.27 days, which explains the decrease in positivity rates (54.24%) of paired sera IgG. A recent study also found that the early IgG samples in patients taken within 7 days of symptoms showed a poor positivity rate (10.7%) (20). Interestingly, the positivity rate of paired sera IgG in pediatric patients taken within 1 week of illness onset was as high as 70.00%, which was higher than those with a gap of longer than 2 weeks. This may be because IgG typically rises after 2 weeks of illness, and we collected the first sera within 1 week of illness, while the second sera was collected after 2 weeks having undergone a positive conversion or significant increase in IgG. However, the detection rate was not consistent with that of BALF PCR. These findings indicate that paired sera collected with less than 2 weeks of disease duration may be of limited value for diagnosing MP infection. Instead, IgM may be a reliable specific indicator of infection in pediatric patients older than 5 years and those with the disease duration of 1–2 weeks.
PCR is accepted as a rapid diagnostic test, and because of its high sensitivity, speed, and specificity, some studies have suggested that PCR tests for MP could replace serology and culture as the “new gold standard” (22). However, PCR is not readily available in many laboratories and may not be suitable for diagnosis in some cases, such as those affected by colonization or prolonged shedding from previous episodes of infection. Spuesens et al. found that by using the PCR method, MPDNA could be detected in 16.2% healthy pediatrics who did not display any respiratory symptoms, and that the MP in the upper respiratory tract could persist for up to 4 months (23). Therefore, it is necessary for physicians to differentiate between a true MP acute infection and a carrier and colonized situation.
An early retrospective study suggested that a high bacterial load was indicative of an MP infection (24), and a threshold of 104 genomic DNA copies per mL can be applied to distinguish clinical infection from carriage (11).To minimize bias induced by cases of colonization in the study, we only enrolled patients showing clinical signs and symptoms compatible with mycoplasma infection, along with MP PCR detection in both NPA and BALF, and defined DNA concentrations ≥1×104 copies/mL as positive MP infection in the NPA samples. The positivity rate of NPA PCR in our study was 64.02%, but in pediatric patients younger than 1 year it was only 30.43%. In those aged between 1–3 years and older than 5 years of age, the positivity rates were 65.22% and 72.72%, respectively, and the detection rates were both consistent with BALF PCR. This showed that the NPA PCR assay may be of good diagnostic value for pediatric patients aged between 1–3 years old and younger than 5 years old.
A previously published research report stated that the detection of IgM antibodies in combination with PCR allowed for a precise and reliable MP diagnosis during the acute phase of the disease (17). In our study, patients were defined as infected with MP if they tested positive for IgM antibodies and/or PCR, and the combined IgM and NPA PCR test showed the highest positivity rates (75.61%). With BALF PCR as the gold standard, the area under the ROC curves for the combined IgM and NPA PCR test was significantly higher. However, our study also found that, even with combined IgM and NPA PCR test, the positivity rate for patients younger than 1 year was only 39.13%, similar to those of NPA PCR, IgM, and paired sera IgG alone. The positivity rates of combined IgM and NPA PCR in pediatric patients aged between 1–3 years old and older than 5 years were 78.85% and 84.85%, respectively, and the detection rates were both consistent with BALF PCR. Combined IgM and NPA PCR was also proved to be of great value for pediatric patients with a duration from onset of 1–2 weeks, which showed a positivity rate as high as 91.11% and detection rates consistent with BALF PCR.
This study has some limitations. Firstly, using bronchoalveolar lavage fluid PCR assay as a gold standard to compare different diagnostic methods is not irrefutable. PCR assays may have false-negative results due to inhibitors in samples or poor technique. Secondly, in our study the median time between the paired sera IgG being taken was only 7.27 days. Lastly, our sample size was relatively small, and further research with larger sample sizes is needed to support our findings.
Conclusions
In conclusion, when evaluating pediatric patients with acute MP infection, our results suggested that, together with compatible clinical history, the combined IgM and NPA PCR is an optimal test for confirming MP infection for pediatric patients aged between 3–5 years and those with a disease onset of less than 2 weeks. NPA PCR or IgM each have prime diagnostic value for pediatric patients over 5 years of age and those with the disease onset of 2–3 weeks. NPA MP-PCR is the most useful test for diagnosing patients aged between 1–3 years. For pediatric patients under 1 year, and those with a disease onset more than 3 weeks, none of the four testing methods in this study are valid and a new diagnostic method should be investigated.
Acknowledgments
Funding: Social Development Projects of Jiangsu Province (grant No. BE2019671); Science and Technology Program of Suzhou (grant No. SS201869); the National Natural Science Foundation of China (grant No. 81870006; 81771676); Jiangsu Provincial Medical Youth Talent (grant No. QNRC2016766); Suzhou Medical Youth Talent (grant No. GSWS2019047); Key Lab of Respiratory Disease of Suzhou (grant No. SZS201714). Suzhou Medical Technology Project of Clinical Key Diseases (grant No. LCZX201809).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Human Ethical Committee of Children’s Hospital of Soochow University (No. 2020LW002). Written consent was obtained from the guardians of each child who participated in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meyer Sauteur PM, van Rossum AM, Vink C. Mycoplasma pneumoniae in children: carriage, pathogenesis, and antibiotic resistance. Curr Opin Infect Dis 2014;27:220-7. [Crossref] [PubMed]
- Defilippi A, Silvestri M, Tacchella A, et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med 2008;102:1762-8. [Crossref] [PubMed]
- Sun H, Chen Z, Yan Y, et al. Epidemiology and clinical profiles of Mycoplasma pneumoniae infection in hospitalized infants younger than one year. Respir Med 2015;109:751-7. [Crossref] [PubMed]
- Tao Y, Tang M, Luo L, et al. Identification of etiologic agents and clinical characteristics for patients suspected of having pertussis in a large Children's Hospital in China. Ann Transl Med 2019;7:443.
- Medjo B, Atanaskovic-Markovic M, Radic S, et al. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: clinical features and laboratory diagnosis. Ital J Pediatr 2014;40:104. [Crossref] [PubMed]
- Pereyre S, Goret J, Bebear C.. Mycoplasma pneumoniae: Current Knowledge on Macrolide Resistance and Treatment. Front Microbiol 2016;7:974. [Crossref] [PubMed]
- Loens K, Van Heirstraeten L, Malhotra-Kumar S, et al. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. J Clin Microbiol 2009;47:21-31. [Crossref] [PubMed]
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004;17:697-728. [Crossref] [PubMed]
- Daxboeck F, Kircher K, Krause R, et al. Effect of age on antibody titer to Mycoplasma pneumoniae. Scand J Infect Dis 2002;34:577-9. [Crossref] [PubMed]
- Thurman KA, Walter ND, Schwartz SB, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis 2009;48:1244-9. [Crossref] [PubMed]
- Williamson J, Marmion BP, Worswick DA, et al. Laboratory diagnosis of Mycoplasma pneumoniae infection. 4. Antigen capture and PCR-gene amplification for detection of the Mycoplasma: problems of clinical correlation. Epidemiol Infect 1992;109:519-37. [Crossref] [PubMed]
- Honda J, Yano T, Kusaba M, et al. Clinical use of capillary PCR to diagnose Mycoplasma pneumonia. J Clin Microbiol 2000;38:1382-4. [Crossref] [PubMed]
- Guo Q, Li HY, Zhou YP, et al. Associations of radiological features in Mycoplasma pneumoniae pneumonia. Arch Med Sci 2014;10:725-32. [PubMed]
- Yan Y, Wei Y, Jiang W, et al. The clinical characteristics of corticosteroid-resistant refractory Mycoplasma Pneumoniae pneumonia in children. Sci Rep 2016;6:39929. [Crossref] [PubMed]
- Chen ZR, Zhang GB, Wang YQ, et al. Soluble B7-H3 elevations in hospitalized children with Mycoplasma pneumoniae pneumonia. Diagn Microbiol Infect Dis 2013;77:362-6. [Crossref] [PubMed]
- Wang Y, Chen Z, Yan YD, et al. Seasonal distribution and epidemiological characteristics of human metapneumovirus infections in pediatric inpatients in Southeast China. Arch Virol 2013;158:417-24. [Crossref] [PubMed]
- Chang HY, Chang LY, Shao PL, et al. Comparison of real-time polymerase chain reaction and serological tests for the confirmation of Mycoplasma pneumoniae infection in children with clinical diagnosis of atypical pneumonia. J Microbiol Immunol Infect 2014;47:137-44. [Crossref] [PubMed]
- Hu CF, Wang CC, Chen SJ, et al. Prognostic values of a combination of intervals between respiratory illness and onset of neurological symptoms and elevated serum IgM titers in Mycoplasma pneumoniae encephalopathy. J Microbiol Immunol Infect 2014;47:497-502. [Crossref] [PubMed]
- Lee WJ, Huang EY, Tsai CM, et al. Role of Serum Mycoplasma pneumoniae IgA, IgM, and IgG in the Diagnosis of Mycoplasma pneumoniae-Related Pneumonia in School-Age Children and Adolescents. Clin Vaccine Immunol 2017. [Crossref] [PubMed]
- Lin LJ, Chang FC, Chi H, et al. The diagnostic value of serological studies in pediatric patients with acute Mycoplasma pneumoniae infection. J Microbiol Immunol Infect 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Waites KB, Balish MF, Atkinson TP. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol 2008;3:635-48. [Crossref] [PubMed]
- Loens K, Ieven M.. Mycoplasma pneumoniae: Current Knowledge on Nucleic Acid Amplification Techniques and Serological Diagnostics. Front Microbiol 2016;7:448. [Crossref] [PubMed]
- Spuesens EB, Fraaij PL, Visser EG, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 2013;10:e1001444. [Crossref] [PubMed]
- Jiang W, Yan Y, Ji W, et al. Clinical significance of different bacterial load of Mycoplasma pneumoniae in patients with Mycoplasma pneumoniae pneumonia. Braz J Infect Dis 2014;18:124-8. [Crossref] [PubMed]


