
Biological variation of metabolic cardiovascular risk factors in haemodialysis patients and healthy individuals
Introduction
The relationship between cardiovascular disease and chronic kidney disease (CKD) is a well-known physiopathological reality: while hypertension and atherosclerosis are some of the most common causes of nephropathy, chronic damage of renal function leads to a vasculopathic state that predispose the development of cardiovascular diseases (1). In epidemiological terms, the final result is twofold: on the one hand, the risk of renal dysfunction is higher in subjects with vascular disease, on the other hand, the risk of cardiovascular events is significantly higher in subjects with CKD than in the general population, particularly in end-stage renal disease (ESRD) patients on haemodialysis (HD) (2).
CKD is associated with a high prevalence of conventional cardiovascular risk factors, such as age, hypertension, diabetes mellitus, dyslipidemia or sedentary lifestyle (1). Since the 2012 update, the European Guide for Cardiovascular Disease Prevention (3) classify CKD patients as very high-risk subjects, in accordance with the recommendations of the Kidney Disease: Improving Global Outcomes (KDIGO) consortium guidelines for the Evaluation and Management of CKD (4). Both guidelines recommend these patients should be included in a systematic approach to risk management that will include, among others, a tight control of blood glucose, blood pressure and the lipid profile.
Nevertheless, it has been reported that conventional risk factors cannot explain the marked increase in cardiovascular morbidity and mortality described in CKD patients, noting that the Framingham score underestimates cardiovascular disease risk (5). Therefore, in these patients, and especially in those on maintenance HD, it will be necessary to consider other emerging or non-conventional risk factors such as inflammation, defined by high C-reactive protein (CRP) concentrations (6), or hyperhomocysteinemia (7); as well as uremia-related metabolic cardiovascular risk factors, such as the disturbances in mineral and bone homeostasis (8). In this context, proper interpretation of the main biochemical cardiovascular risk factors will be key to the appropriate identification and monitoring of high-risk subjects and, for that purpose, biological variation (BV) should be considered.
BV has been defined, in a very simplified way, as an inherent random fluctuation in analyte concentration due to the physiological balance between metabolic turnover and homeostatic regulation. Two components have been described, usually expressed as coefficients of variation (CV): within-subject biological variation (CVI), defined as the average random fluctuation around a homeostatic set-point, and between-subject biological variation (CVG), described as the difference between the set-points of individuals (9). The main applications of this BV data are: to appraise the utility of population-based reference intervals through the index of individuality (II) (9), to set analytical quality specifications (10) and to establish the reference change value (RCV), which allows to evaluate the clinical significance of changes in serial results from an individual (11).
RCV, derived from BV data, is currently considered the best option for the follow-up of patients with chronic pathologies (11). However, if the underlying disease alters the variation around the biomarker homeostatic set-point, the use of RCV estimated from healthy individuals BV data may not be appropriate and could lead to a negative impact on clinical decision making, for example, changing the treatment or performing invasive tests (12).
In a study conducted by our working group and published in 2015 (13), it was noted that ESRD seemed to alter the homeostatic set-point of high-sensitivity serum cardiac troponin T (hs-cTnT), therefore, for an adequate diagnosis of acute cardiovascular events in HD patients, the use of the RCV derived from advance disease-specific BV data was recommended. Based on this observation, the aim of the present study has been to evaluate whether other metabolic cardiovascular risk factors common in the follow-up of HD patients, exhibit similar disturbances in their BV data due to the disease. For this purpose, we estimated the BV data in HD patients in long-term steady-state conditions for serum lipids, calcium, inorganic phosphorus, 25-OH vitamin D, CRP and plasma parathyroid hormone (PTH) and total homocysteine (tHcy). As serum vitamin B12 and folate are main determinants in the metabolism of tHcy, this magnitudes were also included in the study. Likewise, BV data were estimated in healthy subjects for comparison and ultimately, these BV data were used to obtain the II, the RCV and to define analytical quality specifications for imprecision (I), bias (B) and total error (TE).
Methods
Subjects and specimen collection
Estimation of metabolic cardiovascular risk factors BV data was performed on previously obtained samples from two cohorts of 18 stable patients (11 females and 7 males; age range, 31–75 years) undergoing chronic conventional HD three times a week (length of the sessions 3 h), and 11 healthy individuals (8 females and 3 males; age range: 21–50 years) recruited for the assessment of serum hs-cTnT BV. None of the subjects, wherever patients or controls, had a history or pharmacotherapy prescription related to cardiovascular disease, dyslipidemia or secondary hyperparathyroidism.
Samples from HD patients were collected once a month before the HD treatment during a period of 6 months (June–November). Specimens from healthy volunteers were obtained once a week for 5 weeks between September and October. Sampling and analysis were completed in a period of one year.
All samples were collected by one experienced phlebotomist at the same time of day (8:30–10:00) by conventional venipuncture after an overnight fast, with subjects in a sitting position and avoiding venous stasis. Venous blood was obtained using plain tubes and EDTA-containing tubes (Vacuette, Greiner Bio-one, Madrid, Spain). All tubes were centrifuged at 1400 RCF: for serum samples after 30 minutes at room temperature to allow the clot and for plasma samples immediately after extraction to avoid the in vitro degradation of blood components. Both serum and plasma samples were aliquoted and stored at −80 °C within 1 hour, and analysed within a maximum of 12 months.
Table 1 shows the baseline characteristics of HD patients, including comorbidities and pharmacotherapy prescription. Detailed subjects inclusion criteria and management protocols have been described elsewhere (13). The study was approved by the institutional review board and informed consent was obtained from patients and controls.
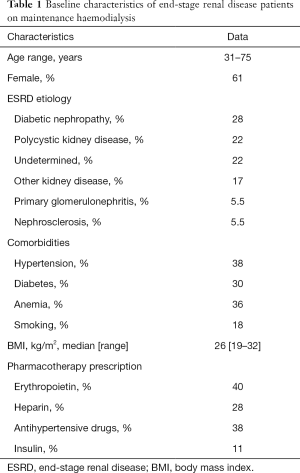
Full table
Analytical methods
Serum CRP, calcium, inorganic phosphorus, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c) through an homogeneous method, were measured using immunoturbidimetric or colourimetric assays, on a Cobas e702 (Roche Diagnostics, Barcelona, Spain). Plasma intact PTH (PTHi) and serum 25-OH-vitamin D, vitamin B12 and folate concentration were analyzed by ECLIA assays on a Cobas e601 (Roche Diagnostics). Non high-density lipoprotein cholesterol (nHDL-c) concentration was calculated by the equation: [nHDL-c = TC-HDL-c].
Plasma tHcy concentrations were measured by reversed-phase high-performance liquid chromatography (RP-HPLC) (Breeze, Waters, Madrid, Spain), by SBD-F (ammonium-7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate) pre-column derivatization and fluorescence detection, as described by Pfeiffer et al. (14).
Analytical performance was strictly standardized to minimize sources of variation. Prior to analysis, internal quality controls (Bio-Rad Laboratories, Madrid, Spain), at two concentrations were analyzed to assess method performance. Assays were also evaluated on a regular basis through participation in national and international external quality surveys (EQAS, Bio-Rad Laboratories).
To remove interbatch analytical variation, all samples from the same group, whether healthy individuals or HD patients, were assayed in duplicate in the same batch. Single lots of reagents and calibrators were employed.
Statistical analysis
The statistical analysis was carried out with SPSS software (23.0) for Windows (SPSS Inc., Chicago, IL). To ensure a steady-state condition in HD patients and healthy individuals during the study period, we used a linear regression analysis with 95% confidence intervals to evaluate the presence of systematic variations in the concentrations of the cardiovascular risk factors under study. None of the subjects showed systematic changes in the concentrations of these magnitudes during the follow-up.
A Levene test was used to confirm the homogeneity of variances, and Cochran and Reed tests were applied for the identification and exclusion of outlying values on the within-subject, between-subject and duplicate data sets. Normal distribution was assessed by the Shapiro-Wilk test and by verification of the straightness of a normal plot. All magnitudes concentrations showed a non-Gaussian distribution in both healthy subjects and HD patients. Mann-Whitney tests were used for median concentrations comparisons and P<0.05 was considered statistically significant.
CV-ANOVA (15) was applied to estimate CVI and analytical CV (CVA). This method is based on the CV transformation with normalization of each subject’s data by dividing by that subject’s mean value. As CV-ANOVA does not provide an estimate of the CVG, this was calculated by a standard nested ANOVA after log-normalization of non-Gaussian variables and retransformation of the estimated component. The 95% confidence intervals (95% CI) for CV were calculated according to the formula from Miller (16), and SDA/SDI ratios were calculated as indicators of the reliability of CVI estimates (17).
BV data estimated in healthy individuals were used to calculate the desirable analytical quality specifications as I =0.5CVI, B =0.25 (CVI2 + CVG2)1/2, TE =1.65 (0.5CVI) + 0.25 (CVI2 + CVG2)1/2; and the II as (CVI2 + CVA2)1/2/CVG. RCV was calculated in both healthy and HD subjects as the probability of a change (two-tailed; Z-score =1.96) at P<0.05 as RCV =21/2×1.96 (CVA2+CVI2)1/2. RCVs were also estimated with the lognormal approach that use the CVTI of non-log transformed data to estimate the σ parameter of the lognormal distribution (15). The asymmetrical limits for the upward value for the lognormal RCV (RCVpos) and for the downward value (RCVneg) were determined as follows: RCVpos = [exp(Zx21/2xσ) –1]×100; RCVneg = [exp(–Zx21/2xσ) –1] ×100.
Previous studies have shown that long-term HD treatment duration may have potential effects on the concentrations of metabolic cardiovascular risk factors and BV data (13). Thus, when evaluating non-acute effects, it could require a longer time for these disturbances to established. Accordingly, HD patients were stratified into two groups: short-term HD (<1 year on HD; eight females, one male; age range 31–75 years), long-term HD (>1 year on HD; three females, six males; HD time range 1–4 years; age range 34–75 years).
Results
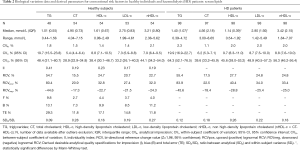
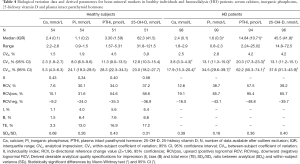
Tables 2-4 show BV components (CVI, CVG) and derived parameters for conventional (serum lipids), non-conventional (serum CRP, vitamin B12, folate and plasma tHcy) and mineral-bone related (serum calcium, inorganic phosphorus, 25-OH-vitamin D and plasma PTHi) metabolic cardiovascular risk factors in HD patients and healthy individuals; as well as the median (interquartile range) concentrations and range, the CVA, the SDA/SDI ratio and the number of available data after the exclusion of outliers. For each magnitude under study, the percentage of excluded data were less than 5%, being evenly distributed among cohorts and subjects; and the SDA/SDI ratios were less than 1.0 for all magnitudes, both in HD patients and healthy individuals.
Full table

Full table
Full table
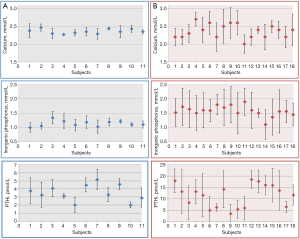
HD patients showed concentrations of serum TG, CRP and inorganic phosphorus, and plasma PTHi and tHcy, significantly higher than those obtained in healthy individuals; while serum HDL-c and 25-OH vitamin D concentrations were significantly lower. According to the 95% CI, CVI and CVG estimated for calcium, inorganic phosphorus and plasma PTHi were significantly higher in HD patients than in healthy individuals and, therefore, RCV derived from these BV data were almost double. As can be seen in Figure 1, healthy individuals showed mild changes in serum calcium, inorganic phosphorus and plasma PTHi concentrations during the follow-up; consequently, the CI of the estimated CVI for these magnitudes, as well as those of the CVG, were relatively narrow. Conversely, the observed variability was significantly higher in HD patients than in healthy subjects, with the median (IQR) for the range of concentrations measured within each subject being 0.30 (0.12) vs. 0.11 (0.05) mmol/L for calcium, 0.54 (0.16) vs. 0.17 (0.07) mmol/L for inorganic phosphorus and 5.66 (2.33) vs. 0.98 (0.42) pmol/L for PTHi, respectively. For the rest of the assessed magnitudes, observed variability during the follow-up was similar in both cohorts.
Finally, comparison of results obtained in short-term and long-term HD patients showed a statistically significant increase in CRP and PTHi concentrations with HD treatment duration, while for TC and HDL-c concentrations a significant decrease was observed. Otherwise, no effect was noted on the BV data.
Discussion
The increased cardiovascular risk in CKD has a multifactorial etiology, being associated with the high prevalence and the cumulative effect of conventional, non-conventional and uremia-related risk factors (4,6-8). Thus, management of the main metabolic cardiovascular risk factors should ultimately aim to reduce the risk of cardiovascular events and to improve survival. In this regard, RCV has proven to be a valuable analytical tool, being used in an increasing number of clinical situations, such the assessment of the prognostic value of changes in cardiac troponin concentrations in HD patients (18). Nevertheless, few specifically designed studies on BV have previously been reported in CKD.
Moreover, despite its undeniable prestige, in the last decade a series of limitations have been identified in the 2014 online BV database, which raise doubts about the quality of the BV data included. In 2018, The European Federation of Clinical Chemistry and Laboratory Medicine Task and Finish Group for the BV Database (TFG-BVD) published the Biological Variation Critical Appraisal Checklist (BIVAC) (19), in order to establish a guide for reliable BV studies. These recommendations have been published after the design and execution of the present study, but still shows a high compliance with defined checklist quality items. Therefore, the data obtained in this study, especially in healthy individuals, could contribute to assess the quality of the data currently available, as well as to enrich the new EFLM biological variation database, at present under development.
The reliability of the BV data generated has been evaluated according to Røraas et al. (17). For the assessment of the CVI estimates, these authors used the CI amplitude and the ratio between analytical (SDA) and within-subject variance (SDI); observing that lower ratios yield narrower CI and increase the power of the estimation, demonstrating that when the ratio is less than 1 the power tends to the unit. According to these criteria; obtained SDA/SDI ratios were below 1 for all magnitudes, both in HD patients and healthy individuals; and CVI also shown relatively narrow 95% CI.
Among the possible limitations of the study, differences in age and number of subjects between healthy and HD cohorts must be considered when direct comparisons of the BV data are performed. However, according to the steady-state conditions in both groups, differences in the duration of observation could have a minor influence on these BV data. Likewise, different sampling periods could lead to seasonal variations that may have influenced the estimation of the BV data for 25-OH-vitamin D, but solar radiation in our latitude (43 °N) is maximum from June to October what would explain the lack of systematic variations observed in both groups. Sample size could be considered relatively small, although it is similar to that of other published studies on BV in both HD patients and healthy subjects (20-22). Finally, to avoid the in vitro degradation of blood components all samples were frozen at −80 °C. This temperature has been shown to preserve most of biochemical magnitudes for at least 12 months, including lipid profile parameters (23), however, this information is not available for all markers studied.
Serum lipid profile observed in patients cohort were in agreement with studies published on dyslipidemia in ESRD (4,24). Likewise, our study shows that a longer period under HD treatment seems to be related to a decrease in TC and HDL-c serum concentrations, possibly due to a deeper deterioration of the nutritional status (25).
CVI estimates for serum lipids derived from both groups are relatively low compared to CVG, with CVI values bellow 10% for all magnitudes with the exception of triglycerides, whose homeostasis regulation seems to be less strict and could be more influenced by physiological or dietary variations. In 2018, EFLM European Biological Variation Study (EuBIVAS) published the BV data for lipids, electrolytes and other biochemical magnitudes in a cohort of 91 healthy subjects (25). According to 95% CI, BV data derived from our study are in agreement with those provided by the euBIVAS group with the exception of HDL-c, for which a slightly higher CVI value has been obtained (8.8% vs. 5.7%).
Despite differences in lipids concentrations, estimated BV data are similar in HD patients and healthy individuals, therefore RCV derived from healthy subjects could be applied for the lipid profile management in HD patients. Due to the non-Gaussian distribution, the use of the asymmetric RCV will provide a more precise evaluation of treatment response for dyslipidemia both in the general population and in HD patients. Accordingly, for LDL-c and nHDL-c, an adequate adherence to lipid-lowering therapy would be evidenced by a reduction in their serum concentration of at least 21.5% and 24.3%, respectively.
Regarding non-conventional cardiovascular risk factors, during follow-up most HD patients showed a serum CRP median concentration up to 8 times higher than that of healthy subjects; observing also a significant increased in CRP concentrations with a longer period under HD treatment. This evidences that ESRD is characterized by a chronic inflammatory state, as it has been previously reported (6). Meanwhile, plasma tHcy concentrations observed in HD patients were significantly higher than that of healthy individuals, which is consistent with the data collected in the literature (6,7,26). It should be noted that no subject, whether HD patients or healthy individuals, had serum folate or vitamin B12 deficiency concentrations.
CVI data estimated for serum CRP in both HD patients and healthy individuals are more than twice that obtained for the other metabolic cardiovascular risk factors under study. Conversely, CVI data derived for plasma tHcy are relatively low compared to CVG and similar to that estimated for serum folate and vitamin B12, an expected similarity given their close physiopathological relationship. BV data generated for these four magnitudes are in accordance with those collected in the 2014 online BV database (27).
As for serum lipids, the lack of differences between the BV data estimated in both cohorts suggests that RCV derived from healthy subjects could be properly used in the monitoring of chronic inflammation and hyperhomocysteinemia in HD patients.
Chronic kidney disease-mineral bone disorder (CKD-MBD) is a systemic condition that manifests as a disruption of normal plasma concentrations of inorganic phosphorus, calcium, vitamin D, fibroblast grown factor 23 and PTHi; bone abnormalities and extraskeletal calcifications (8,28).
Most HD patients accomplished the KDIGO recommendations for adequate serum calcium and inorganic phosphorus, while plasma PTHi concentrations, although significantly higher than in healthy individuals, were below those considered sufficient to maintain adequate bone remodeling in ESRD (29). Interestingly, our data show that a longer duration of HD treatment seems to be related to an increase in plasma PTHi concentration, possibly due to a decrease in vitamin D receptors at the parathyroid (29).
According to the 95% CI, the CVI derived from healthy individuals are in accordance with those published by the euBIVAS study for total serum calcium and inorganic phosphorus (26), while the estimated CVI in HD patients are significantly higher; suggesting a tight homeostatic regulation of mineral metabolism that seems to be affected by ESRD. Plasma PTHi CVI estimated for HD patients (20.3%) is also significantly higher than that generated for healthy individuals (11.3%), indicating that CKD modifies the variation around the homeostatic set-point, as has been previously noted by Gardham et al. (30). On the contrary, no differences are observed between BV data estimated for 25-OH vitamin D in HD patients and healthy individuals.
Plasma PTHi CVI estimates for HD patients were in between those previously published by Gardham et al. (30) (CVI =25.6%) and Cavalier et al. (20) (13.8%) in a 6-week follow-up studies of 22 and 17 stable HD patients, respectively. Likewise, BV data generated for healthy individuals differ from those previously reported by Ercan et al. (21) in 20 healthy individuals with equivalent follow-up period and, to a lesser extent, from those estimated by Lutsey et al. (22) on 160 Atherosclerosis Risk in Communities (ARIC) study participants; who obtained higher CVI estimates for plasma PTHi and lower for 25-OH vitamin D. Potential reasons for these discrepancies could be related to the different number of subjects, frequency of sampling or statistical treatment of data, such as our estimation of BV components by the novel CV-ANOVA method after outlier exclusion.
As a consequence of the differences in CVI data, the use of RCV derived from healthy individuals for the management of HD patients would lead to an increase in false positive changes, with the consequent repercussion in clinical decision making (12). Accordingly, the use of the asymmetric RCV derived from advanced disease-specific BV data seems to be more appropriate for an adequate clinical interpretation of mineral-bone metabolism related magnitudes in HD patients.
In summary, relatively low CVI in relation to CVG for all magnitudes have been observed in both healthy individuals and HD patients. Unlike the rest of metabolic cardiovascular risk factors on assessment, BV data for mineral-bone metabolism related magnitudes seems to be affected by ESRD; therefore, the use of appropriate asymmetric RCV values is recommended for the management of CKD-MBD in HD patients.
Acknowledgments
The authors thank the study volunteers and the San Agustin University Hospital Nephrology Service for their invaluable collaboration.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board, the Comité de Ética de la Investigación del Principado de Asturias (reference number: 87/14).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rangaswami J, Bhalla V, Blair JEA, et al. American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019;139:e840-78. [Crossref] [PubMed]
- US Renal Data System, USRDS 2017 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda MD, 2017.
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2016;37:2315-81. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150.
- Poli FE, Gulsin GS, McCann GP, et al. The assessment of coronary artery disease in patients with end-stage renal disease. Clin Kidney J 2019;12:721-34. [Crossref] [PubMed]
- Fonseca FA, Izar MC. High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities. Clinics (Sao Paulo) 2016;71:235-42. [Crossref] [PubMed]
- Martella BM, Veiga GRL, Alves BCA, et al. The importance of homocysteine levels in the prognosis of patients with chronic renal disease and in hemodialysis patients. J Bras Patol Med Lab 2018;54:170-6. [Crossref]
- Vervloet MG, Massy ZA, Brandenburg VM, et al. Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2014;2:427-36. [Crossref] [PubMed]
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409-37. [Crossref] [PubMed]
- Panteghini M, Sandberg S. Defining analytical performance specifications 15 years after the Stockholm conference. Clin Chem Lab Med 2015;53:829-32. [Crossref] [PubMed]
- Bugdayci G, Oguzman H, Arattan HY, et al. The use of reference change values in clinical laboratories. Clin Lab 2015;61:251-7. [Crossref] [PubMed]
- Ricós C, Iglesias N, García-Lario JV, et al. Within subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem 2007;44:343-52. [Crossref] [PubMed]
- Corte Z, García C, Venta R. Biological variation of cardiac troponin T in patients with end-stage renal disease and in healthy individuals. Ann Clin Biochem 2015;52:53-60. [Crossref] [PubMed]
- Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999;45:290-2. [Crossref] [PubMed]
- Røraas T, Stove B, Hyltoft Petersen P, et al. Biological variation: the effect of different distributions on estimated within-person variation and reference change values. Clin Chem 2016;62:725-36. [Crossref] [PubMed]
- Miller EG. Asymptotic test statistics for coefficient of variation. Comm Stat Theory & Methods 1991;20:3351-63. [Crossref]
- Røraas T, Petersen P, Sandberg S. Confidence intervals and power calculations for within-person biological variation: effect of analytical imprecision, number of replicates, number of samples, and number of individuals. Clin Chem 2012;58:1306-13. [Crossref] [PubMed]
- Sandoval Y, Herzog CA, Love SA, et al. Prognostic Value of Serial Changes in High-Sensitivity Cardiac Troponin I and T over 3 Months Using Reference Change Values in Hemodialysis Patients. Clin Chem 2016;62:631-8. [Crossref] [PubMed]
- Aarsand AK, Røraas T, Fernandez-Calle P, et al. The Biological Variation Data Critical Appraisal Checklist: A Standard for Evaluating Studies on Biological Variation. Clin Chem 2018;64:501-14. [Crossref] [PubMed]
- Cavalier E, Delanaye P, Moranne O. Variability of new bone mineral metabolism markers in patients treated with maintenance hemodialysis: implications for clinical decision making. Am J Kidney Dis 2013;61:847-8. [Crossref] [PubMed]
- Ercan M, Deniz E, Avci E, et al. Determining biological variation of serum parathyroid hormone in healthy adults. Biochem Med (Zagreb) 2019;29:030702. [Crossref] [PubMed]
- Lutsey PL, Parrinello CM, Misialek JR, et al. Short-term variability of vitamin D-related biomarkers. Clin Chem 2016;62:1647-53. [Crossref] [PubMed]
- Ugwuzumba PC, Nwankpa P, Emengaha FC, et al. Influence of Freeze-Thawing and Storage on Human Serum Lipid Analytes. Int J Curr Microbiol App Sci 2018;7:3287-94. [Crossref]
- Sabatino A, Regolisti G, Karupaiah T, et al. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr 2017;36:663-71. [Crossref] [PubMed]
- Aarsand AK, Díaz-Garzón J, Fernandez-Calle P, et al. The EuBIVAS: Within- and Between-Subject Biological Variation Data for Electrolytes, Lipids, Urea, Uric Acid, Total Protein, Total Bilirubin, Direct Bilirubin, and Glucose. Clin Chem 2018;64:1380-93. [Crossref] [PubMed]
- Yeh EL, Huang YC, Tsai SF, et al. Relationship between plasma levels of homocysteine and the related B vitamins in patients with hemodialysis adequacy or inadequacy. Nutrition 2018;53:103-8. [Crossref] [PubMed]
- Minchinela J, Ricos C, Perich C, et al. Biological variation database, and quality specifications for imprecision, bias and total error (desírable and minimum). The 2014 update. Available online: https://www.westgard.com/biodatabase1.htm, 2018 (accessed 23 January 2018).
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO 2017 clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Kidney Int Suppl 2017;7:1-59. [Crossref]
- Fujii H. Association between parathyroid hormone and cardiovascular disease. Ther Apher Dial 2018;22:236-41. [Crossref] [PubMed]
- Gardham C, Stevens PE, Delaney MP, et al. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol 2010;5:1261-7. [Crossref] [PubMed]


