The use of algeness in the face and neck: a safe, alternative filler for cosmetics and reconstruction
Introduction
Filler treatments have undoubtedly become one of the most useful and elegant tools for facial rejuvenation worldwide. Beyond their indication in cosmetic procedures, reaching from facial wrinkles to facial sculpting, they can offer an alternative therapeutic option in various functional disorders in the head and neck area. Basic and advanced dermal filler procedures require safety and proficiency that is derived from knowledge of anatomy, proper injection techniques and correctly chosen products for each treatment area (good flow characteristics, duration, hydration and others).
In this article, different interdisciplinary applications of an alternative, 100% natural, degradable filler in the face and neck region are demonstrated. Algeness is an absorbable injectable gel composed of agarose. In the 2.5% and 3.5% agarose concentrations, 0.5% and 0.4% noncrossed-linked hyaluronic acid is added respectively, simply for lubrication and ease of injection. Agarose is a unique material with special rheological characteristics and gel forming capability (1-3). Its hydrocolloid nature allows very precise injections with immediately visible results. As the product is non-hydrophylic and does not cause mid- or long-term oedema of the surrounding tissues, only its slow resorption has an impact over time on the clinical result. It is effective for skin fold and scar corrections, but can also be used in augmentation of deeper cutaneous tissue (4).
Dr. Leonard Miller, a noted plastic surgeon in the US, first discovered the wealth of data and information on agarose gels derived from red algae (5).
Methods
The clinical use of Algeness has expanded greatly during the last several years. In order to understand the reason behind the positive effects and success of this injectable, one must be introduced to its biochemical composition and mechanism of action. It contains a gel of biocompatible, highly purified agarose in different concentrations (1%, 1.5%, 2.5%, and 3.5%), sodium and water for injectable preparations. The 2.5% and 3.5% products include also non cross-linked hyaluronic acid in very low concentrations (0.5% and 0.4% each) Algeness fillers show long lasting results over to 12 months demonstrated by clinical studies. The low-density Algeness 1% and 1.5% have a duration of 4–8 months or more and the higher density preparation of 2.5% and 3.5% a duration of 8–12 months or more (4,5).
Agarose is a polymer, extracted from the species of red algae known as agarophytes (derived from the genus Gelidium and Pterocladia) and one of the two principal components of agar. It is purified from agar by removing agar’s other component, agaropectin (6). The high-strength gelling properties of agar are provided by agarose and the viscous properties by agaropectin (7).
Chemically, agarose is a neutral polysaccharide of a long chain of disaccharide units, formed by two rings of 6 atoms. On each ring there is one oxygen and 5 carbon atoms and one ring contains only one OH group, while the other contains three. In presence of water, Agarose forms hydrocolloids. Moreover, it is free from toxicity of microorganisms and free from impurities. Algeness is colorless and painless during injection due to isotonic and isosmotic characteristics (4).
Algeness is contained in a sterile syringe (not pre-mixed with any anesthetics), connected with a hub to an empty, second syringe. This allows for mixing of the gel with 0.2 mL lidocaine 1–2%. It is recommended to move the product back and forth from one syringe to the other at least twenty times for local anesthesia if desired, and to obtain a better consistency prior to injection. The polysaccharide chains spontaneously align over time, increasing the product’s viscosity. Mobilizing the product by exchange between the two syringes restores optimal injectability. Agarose is composed of a three dimensional “plastic reticulum” that is slowly absorbed (8).
It is a well-tolerated injectable, held in place by the macrophage infiltrate, showing no migration.
Algeness can be positioned from the subcutis to the mid or deep dermis, according to the treatment layer and the chosen concentration of Agarose. However, intradermal injection is not recommended. Once the gel is injected into the subcutis it provides supplementary viscoelasticity, volume and shaping to the intracellular matrix of the connective tissue. This is the main mechanism of action, that allows treating cases of connective tissue deficits, dermal atrophy, traumatic lesions, correction of wrinkles, skin folds, scars and also deep tissue augmentations.
Prior to injecting the product, the application area must be disinfected, to minimize the infection risks.
Overcorrections should be avoided. Additional corrections can be required to improve the functional or aesthetic outcome after a period of one week to ten days.
For safety reasons the injection must be performed slowly, by applying a constant, gentle pressure on the syringe plunger to overcome the initial extrusion force of the injection. Proper consideration must be given to precise deposition of the product with an atraumatic technique at the lowest possible risk. The treated area should be gently massaged immediately after the injection, to achieve a homogenous distribution of the filler. This also allows for shaping the injectable material. As with any injectable material, Algeness should not be injected intravascular to avoid embolisation, vessel occlusion and necrosis. Serious adverse events are very rare. Swelling and skin erythema around the application site resolves spontaneously within a few hours to a week. Agarose is a completely biodegradable product. It is removed from the injection site by a process of macrophage phagocytosis and intracellular metabolization in the pentose cycle. This very special ability is the reason that gives Algeness its safety, but it can be also fully dissolved by injecting vitamin C (250–500 mg/mL in 0.1–0.2 mL aliquots), saline solution or hyaluronidase into treated areas (1).
Results
A variety of head, face and neck disorders can be treated with this material, that allows to volumize soft tissue instantly. The first author of this article first injected Algeness in a wide indication range with focus in the field of functional disorders. Others of the authors have a wide variety of experience with aesthetic conditions.
Very promising functional indications with immediate and long lasting results are.
The management of periprosthetic leakage of liquids in patients rehabilitated with a Provox prosthesis after total laryngectomy, due to prevention of recurrent aspiration pneumonia
Most of the seven subjects treated from the first author, underwent a total laryngectomy because of laryngeal cancer, followed by adjuvant radiation and chemotherapy. In all patients, voice functions where replaced by a voice prosthesis “Provox”, placed in an iatrogenic tracheo-esophageal fistula created during operation. This prosthesis is a one-way medical grade silicone air valve that allows air to pass from the trachea to the esophagus when the patient covers the tracheostoma, so that redirected air vibrations on the esophageal tissue can produce a sort of new “voice”.
Mostly after the first post-op year and after radiation, patients with Provox have to face some practical and functional problems with their silicone prosthesis. Due to slow material destruction because of candida, bacteria and tissue atrophy around the Provox, the valve flap of the voice prosthesis does not close properly anymore. This causes leakage mainly of saliva and fluids while drinking, as they enter the trachea directly, due to the post-laryngectomy anatomical changes. This situation causes worrisome coughing during drinking or swallowing, with the danger of aspiration pneumonia. Generaaly, this is an indication to change the voice prosthesis.
A swallowing test is performed in order to locate the leakage. If saliva or fluid flows from the central part of the Provox, replacement is indicated. In cases of a peripheral leakage (around the Provox) or mostly at the upper part of the Provox, injections with fillers can solve the problem easily Algeness proved to be an excellent choice of filler for this indication (Figure 1). Immediately after the injection of a small amount, mostly 0.3–0.5 mL of Algeness 2.5%, the result can be controlled with a new swallowing test with some liquid as water, milk, etc. (Figure 2). Dysphagia or pain symptoms were not reported and all patients experienced an improvement in their quality of life, having the opportunity to drink and eat without the possible danger of a pneumonia. Moreover, all patients avoided an unpleasant Provox replacement and reported being symptom free from 5 to 13 months. Prior to injection tracheal reflexes and coughing can be controlled with the use of xylocaine spray directly on the Provox area.
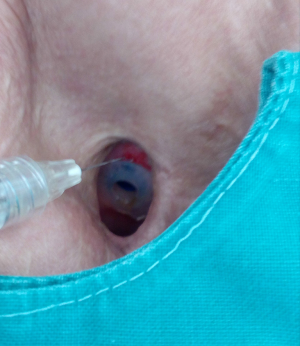
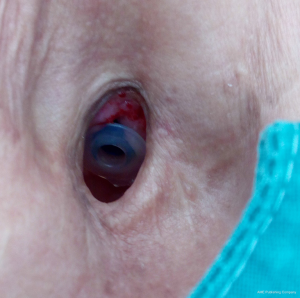
Injection laryngoplasty
A very promising indication is the vocal fold injection for unilateral vocal fold paralysis. Algeness 2.5%, with its medium volumizing capability, and the low density Algeness 1.5% offer a very safe solution, improving glottal closure insufficiency with hoarseness of voice. Because of its non-hydrophilic consistency, Algeness is safer than pure hyaluronic acid, which is hydrophilic with water absorption from surrounding tissues, which could lead to dyspnea, prolonged intubation or even tracheotomy. Optimally, the injections can be performed under general anesthesia and microlaryngoscopic assistance for a precise application.
Moreover, laryngeal injections are also medically necessary for the management of voice loss and aspiration in cases where unilateral vocal cord paralysis is anticipated to be short-term (for example in cases of post-thyroidectomy unilateral paralysis, where the recurrent nerve is known to be intact). Irrespective of the exact usage of the product, the basic prerequisite for laryngeal treatments is a meticulous injection technique, due to dyspnea danger, starting with very low filler amounts. Algeness is a perfect tool, without hydrophilic characteristics and delivering an immediate control of the treated area. No other injectable alternatives combine to this degree high safety with immediate and long- lasting results.
Cosmetic indications in the head and neck area
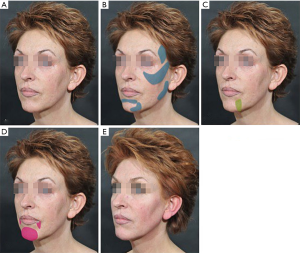
Ageing of the face and neck, is a catabolic tissue process that leads to morphological and physiological changes. This anatomical involution is translated in volume loss of bone and fat, wrinkle formation, elastin and collagen reduction. Moreover, congenital or acquired deformities of the nose, ears, lips, facial contours and scars may affect patients and make an iatrogenic intervention for aesthetic corrections necessary. In all these cases, Algeness and its ability for immediately visible results offers an excellent alternative for contouring, molding and re-shaping.
Non-surgical rhinoplasty either for simple or more severe deformities is a very attractive procedure to many patients that are not prepared for a surgical intervention or wish no downtime. The central, prominent position of the nose on the face and its high risk regions such as the nasal tip and glabella, turn this indication into a field for rather experienced injectors. Usually the nasal tip is used as an entrance point to introduce a 25 G cannula. This manoeuvre is well-tolerated without anesthesia. Indicated products are Algeness 2.5% and 3.5%, injected deep supra periosteal and linear retrograde, while pressing tightly on each side of the nose to occlude vessels and avoid inter arterial injection (4). Complication is reduced by good technique (slow injection, aspiration prior to injection, use of a cannula if possible) and awareness of proximal anatomy. As the ophthalmic artery terminates into the smaller superior trochlear and dorsal nasal artery, intravascular injection of fillers may lead to occlusions of the internal carotid and retinal arteries, causing brain infarction and blindness (9). The signature clinical feature of ocular embolism is direct excruciating ocular pain and visual impairment (10). There might be no other area in the face where prevention is that important, when considering that even urgent ophthalmological interventions remain mostly unsuccessful. An alar and nose tip embolization may clinically cause ischemic phenomenona such as persistent erythema or dermal necrosis.
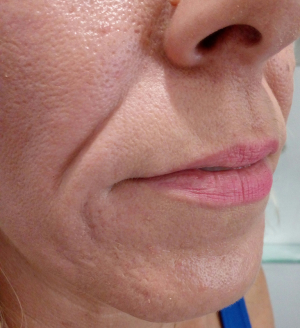
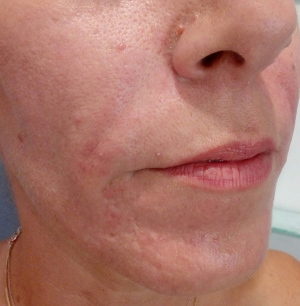
Deepening of the nasolabial folds is one of the midface aging signs (Figure 3). The higher density preparations Algeness 2.5% or 3.5% can be injected in Ristow’s space, under the ala of the nose, beneath the elevator muscle of the lower lip, thereby compensation premaxillary bone deficit. If an injection is performed linear retrograde, just medial to the fold, in the immediate subdermal plane, a small quantity of Algeness 1% may suffice to raise the surface of the lip. If the cheek is compressed just lateral to the crease during injection, there is less chance of the product inadvertently spreading from the fibrous lip subcutis into the softer cheek subcutis, which would increase undesirable nasolabial fullness. It may help to prevent inadvertent intra-arterial spread of the injectable. Giovanni Montealegre Gomez from the National University of Colombia, Bogota, evaluated the safety and efficacy of Algeness versus Juvederm® for this specific indication, performing a double blind study. Each subject received treatments, one substance in one nasolabial fold and the other in the contralateral nasolabial fold. The study proved that both products have comparable effects on duration and safety. Algeness application was described as more painful, a problem that can be easily resolved when adding 1% lidocaine without epinephrine to the syringe (5). Similar to the potential dangers of the nasal anatomy, awareness has to be given to the nasolabial arteries when volumizing this region. Arterial occlusion in this area can lead to necrosis, scarring or disfigurement of the nasal ala, tip, nasolabial fold and upper lip (11). Any practitioner should be mindful of the facial artery’s terminal part, the angular artery, running close to the skin surface at the nasolabial fold. Therefore injections must be performed in a linear threading technique and after having aspirated (Figure 4).
The recommended products for lip augmentation are Algeness 1.5% or 2.5%, that are injected slowly, deep and using a linear retrograde technique to evenly increase volume of the two lateral upper lip aesthetic units and both lower lip aesthetic units. A small bolus injection of 0.1 to 0.15 mL can nicely increase projection and eversion of the central upper lip region.
Post treatment molding or massage is of great importance and should always be considered, especially when injecting lips (2), to avoid palpability of macrophage conglomerates around the product. Scarano et al. published in 2009 the results of a 3 year follow up study of 68 cases that had undergone lip augmentation with agarose gel, proving it as a reliable and predictable treatment option (3). Deep subcutaneous deposition of the filler is least likely to affect natural lip mobility. Injection under the dry vermilion directly increases visible lip volume and projection. Injection under the wet vermilion indirectly projects and everts the lip when there is sufficient dental support. We recommend not to inject Algeness intradermally. Injection of a bolus of more than 0.1 ml is not recommended to avoid palpability and lump formation. Serial injection of boluses has been observed to work well and linear retrograde injections with a 25 cannula work well, too. Generous remodeling decreases the risk of palpability and is strongly recommended, more so than when hyaluronic acid is used.
Mandibular and chin enhancement treatments can make a significant difference to the entire face adding volume, refining and improving the definition of these lines. For these indications Algeness 2.5% or 3.5% can be used for deep submuscular and intramuscular injections, or 1.5% for deep subcutaneous applications, while deep injections into muscle tissue may be less painful when a needle is used, for safety reasons injections are best performed slowly and with the needle in motion. For subcutaneous injections, a linear retrograde injection technique with a cannula may decrease the risk of bruising and intravascular injections. A comprehensive knowledge of the anatomy requires outlining the facial vessels as the most dangerous structures, when it comes to injecting these zones.
The zygomatic region can be volumized with Algeness 2.5% or 3.5% “close to the bone”, as there is no periosteum in this area. The submalar region on the parotid fascia and the anteromedial cheek hollows can be injected subcutaneously. Algeness 1.5% and use of a cannula are recommended. Respecting the recommended tissue layer, protects the practitioner by avoiding key vessels like the facial artery, the transverse facial artery, the infraorbital, the buccal branch of the maxillary and the zygomatic branch of the lacrimal artery. In all midface indications, Algeness is placed slowly and molded carefully post injection to optimize the shaping.
The tear trough deformity is a challenging area in facial rejuvenation. It is a concave depression of the lower eyelid, located inferior to the orbital fat. Etiologically natural aging and inherited anatomic variations are responsible for this fatigued patient appearance (12,13). According to Flowers, descent of the cheek, facial volume loss, underdevelopment of the infraorbital malar complex and a muscular defect between the orbicularis muscle and angular head of the quadratus labii superioris muscle are responsible for the formation of tear trough deformity (14,15). In these cases, Algeness 1% and 1.5% provide a natural and immediate solution. Limited transient swelling may occur, but the hydrocolloid feature of this filler makes it less likely to cause mid- or long term oedema, compared to other injectables with hydrophilic characteristics. After evaluation and marking of the tear trough, prilocaine mixed with lidocaine topical anesthetic ointment is applied to the treated zone. Injections are performed carefully, in order to avoid superficial application, either with needle or cannula, deep in the supra-periosteal plane, reducing the visibility of the product (15). Algeness is placed slowly in small amounts, between the insertion of the medial orbicularis muscle at the maxilla and continues laterally and inferior to the orbicularis retaining ligament. A gentle post injection massage helps to disperse visible irregularities. Subcutaneous injection over the orbital margin holds a high risk of visibility, palpability and an unnatural aspect on facial animation. We strongly recommend against it.
Another area that can successfully be treated with Algeness 2.5% or 3.5% is the temple in cases of depression. The material is placed deep in the temporalis muscle origin, anteriosuperiorly in the temporal fossa, thereby avoiding the superficial temporal artery. It is least painful to do this by needle injection after finding contact with the bone and performing an aspiration test. After a slow bolus injection, it is easy to remodel the injectable in the temporalis muscle. It is difficult to remodel filler after perpendicular injection in the fibrous zone on the temporal crest. Hiding a sharp temporal crest can be performed by injecting Algeness 1.5% subcutaneously with a cannula. By limiting the injection to the immediate subdermal plane, the superficial temporal vessels, associated with the superficial temporal fascia, can be avoided.
After Algeness treatments patients often experience sensitivity, redness, swelling and tenderness near the injection site. All of these conditions are temporary and disappear within a few hours or a couple of days. Major complications, such as inflammatory nodules, granuloma, biofilm or infection are rare for most injectables, but have not been reported so far following an Algeness treatment. Potential vessel occlusions may always occur and have generally a similar symptomatology: discoloration in the form of blanching, prolonged erythema, ecchymosis, intense pain. This is, followed by ischemia and tissue necrosis unless there is a prompt embolization or compression treatment (16). In exceptional situations Algeness can be dispersed by warm saline solution, Vitamin C solution (250–500 mg/mL) or by injecting hyaluronidase if necessary. Basic complication prophylaxis rules include aspiration before injecting, application during a retrograde withdrawal and with small aliquots of material. Using epinephrine should be avoided, so that the cause of blanching can be detected immediately. Moreover, occluding the origin of important vessels manually with the non-dominant finger can minimize the embolization risk (11).
Conclusions
For the first time in face and neck treatments, a 100% natural, biocompatible and biodegradable filler is available for safe and mastered injections (4). Algeness is a volumizing injectable, agarose based filler with unique properties, such as immediately visible results, little to no migration (especially important in the cheek area or in intra-laryngeal placements) and biocompatibility with the human body (17). Cross-linked synthetic chemicals (like BBDE) associated with Hyaluronic acid (HA) fillers are not contained in the preparation. One more advantage is great tissue tolerability with an insignificant immunological reaction (18). Due to the absence of foreign body reactions it is accompanied by minimal irritation and inflammation. Clinical studies demonstrate long lasting results over 12 months. All these characteristics allow treatments over a wide range of indications: beginning from aesthetic disorders to compensate bone mass, subcutaneous and deeper volume loss, reaching to functional disorder treatments of the Face and Neck, like injection laryngoplasty or myringoplasty. In general conclusion, its impressive shaping capabilities turn Algeness into an ideal lifting, shaping and face contouring tool (Figure 5). Knowing how to dispense this volume enhancer, what danger zones to avoid, and the correct managing of potential complications, ensure that the physician is optimally prepared to deliver satisfying clinical outcomes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Dr. Kinney is a member of the Board of Directors, one of the developers of the product and an investor in the company. Dr. Vandeputte is investor in the company. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fu XT, Kim SM. Agarase: Review of Major Sources, Categories, Purification Method, Enzyme Characteristics and Applications. Mar Drugs 2010;8:200-18. [Crossref] [PubMed]
- Shab A. Lippenaugmentation mit der neuen Fillergeneration Agarose-Gel. Face 2016;2:17-8.
- Scarano A, Carinci F, Piattelli A. Lip augmentation with a new filler (agarose gel): a 3-year follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e11-5.17.
- Shab A, Shab C. Agarose gel-high patient satisfaction of a full-facial volume augmentation. Aesthetic Medicine 2018;3. Available online: www.algeness.com
- Advanced Aesthetic Technologies, Algeness Agarose Gel Dermal Filler Information. Available online: www.algeness.com. Information letter for Algeness.
- Jeppsson JO, Laurell CB, Bi F. Agarose gel electrophoresis. Clin Chem 1979;25:629-38. [Crossref] [PubMed]
- Scarano A. Ringiovanimento dei tessuti molli periorali con agarose gel. Dent Clin 2009;2:5-13.
- Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg 2009.Suppl 2:1612-9. [Crossref] [PubMed]
- Carle MV, Roe R, Novack R, et al. Cosmetical facial fillers and severe vision loss. JAMA Ophthalmol 2014;132:637-9. [Crossref] [PubMed]
- Chen Y, Wang W, Li J, et al. Fundus artery occlusion caused by cosmetic facial injections. Chin Med J (Engl) 2014;127:1434-7. [PubMed]
- Emer J, Waldorf H. Injectable neurotoxins and fillers: There is no free lunch. Clin Dermatol 2011;29:678-90. [Crossref] [PubMed]
- Espinoza GM, Holds JB. Evaluation and treatment of the tear trough deformity in lower blepharoplasty. Semin Plast Surg 2007;21:57-64. [Crossref] [PubMed]
- Jiang J, Wang X, Chen R, et al. Tear trough deformity: different types of anatomy and treatment options. Postepy Dermatol Alergol 2016;33:303-8. [Crossref] [PubMed]
- Flowers RS. Tear trough implants for correction of tear trough deformity. Clin Plast Surg 1993;20:403-15. [PubMed]
- Sharad J. Dermal Fillers for the Treatment of Tear Trough Deformity: A Review of Anatomy, Treatment Techniques, and their Outcomes. J Cutan Aesthet Surg 2012;5:229-38. [Crossref] [PubMed]
- Hirsch RJ, Lupo M, Cohen JL, et al. Delayed presentation of impending necrosis following soft tissue augmentation with hyaluronic acid and successful management with hyaluronidase. J Drugs Dermatol 2007;6:325-8. [PubMed]
- Fernández-Cossío S, León-Mateos A, Sampedro FG, et al. Biocompatibility of Agarose Gel as a Dermal Filler: Histologic Evaluation of Subcutaneous Implants. Plast Reconstr Surg 2007;120:1161-9. [Crossref] [PubMed]
- Rubin MG. Treatment of nasolabial folds with fillers. Aesthet Surg J 2004;24:489-93. [Crossref] [PubMed]