
γ-aminobutyric acid and glutamate/glutamine alterations of the left prefrontal cortex in individuals with methamphetamine use disorder: a combined transcranial magnetic stimulation-magnetic resonance spectroscopy study
Introduction
Methamphetamine is one of the most commonly used amphetamine type stimulants (ATS) worldwide, and constitutes a serious burden on global health. Both preclinical and clinical research have shown that methamphetamine may cause long-lasting impairment in the brain (1,2). Despite sustained efforts to develop treatments for methamphetamine addiction, there is no effective method except for psychological intervention.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive technique with demonstrated effectiveness in the treatment of psychiatric disorders (3). In recent years, there have been several investigations using rTMS to treat substance addiction (4). Our preliminary results demonstrated that rTMS could reduce craving and improve cognitive function after a one week intervention (5). However, its mechanisms of action have not yet been unravelled. Neuroimaging tools can improve our understanding of how rTMS works in treating addiction by examining structural, fibrous, functional connectivity alterations after intervention (6-9). In addition, proton magnetic resonance spectroscopy (1H MRS), can be used to noninvasively measure concentrations of key metabolites in specific brain regions (10,11).
Conceptually, excitatory glutamatergic hyperactivity and downregulation ofγ-aminobutyric acid (GABA) inhibitory neurotransmission are important mechanisms of addiction (12,13). Both GABA (GABAA and GABAB receptors) and glutamate (metabotropic glutamate receptors, mGluRs) are potential targets for addiction treatment, and have been investigated in preclinical studies (14,15). Increased glutamate has been reported in the frontal white matter pathways of abstinent methamphetamine dependent subjects (16), while methamphetamine users with less than one month of abstinence show reduced frontal gray matter glutamate and glutamine (Glx, glutamate is a precursor, glutamine is a neurotransmitter) (17). In addition, lisdexamfetamine (L-lysine-d-amphetamine), a prodrug of the psychostimulant d-amphetamine, which is used for treating attention deficit hyperactivity disorder (ADHD), has been shown to decrease Glx in DLPFC (18). Previous evidence has also revealed that glutamate and GABA are found in especially high concentrations in the prefrontal cortex (19,20). It has been speculated that rTMS over the prefrontal cortex may affect glutamatergic and GABA-ergic neurotransmission and induce cortical changes in glutamate/GABA levels. This assumption is supported by the observation that prefrontal rTMS can increase cortical Glx levels in healthy individuals (21) and improvement in a case with treatment refractory substance use disorder (22). Although TMS affects neurometabolites in cortical circuits, it is unknown whether these changes mediate therapeutic response in methamphetamine addiction.
The objective of this study is to investigate neurochemical alterations in the concentrations of Glx, GABA and other key metabolites [n-acetyl-aspartate (NAA), choline (Cho), myo-inositol (mI) and creatine (Cr)] using proton magnetic resonance spectroscopy (1H MRS) in the left dorsolateral prefrontal cortex (DLPFC) of patients with methamphetamine dependence treated with real or sham rTMS. The left DLPFC is a widely accepted location for rTMS intervention in addiction (23). We hypothesize that metabolites levels will change in the DLPFC after 4 weeks of rTMS intervention. Furthermore, we expect that alterations in these concentrations will correlate with craving and cognition changes in methamphetamine dependent patients.
Methods
Participants
Fifty subjects (20 females) who met the criteria for severe methamphetamine use disorder based on the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) were recruited from the Shanghai Drug Rehabilitation Center. Inclusion criteria included more than 9 years of education, aged 18–49 years, and normal vision and audition. Exclusion criteria included serious physical or neurological illness, any other psychiatric disorder according to DSM-5 criteria, and contraindications to rTMS. All participants were interviewed by a psychiatrist and completed a self-administered case report form, which included socio-demographic and drug use characteristics. Participants were unmedicated throughout rTMS treatment.
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board and the ethics committee of Shanghai Mental Health Center. All participants participated voluntarily and provided written consent after understanding the whole procedure.
rTMS protocol
Participants were randomized to receive either real or sham rTMS intervention. All patients completed 20 sessions of rTMS over the left DLPFC over a 4-week period using the MagPro X100 device (MagVenture, Farum, Denmark). Individual sessions consisted of 5 min (900 pulses; 3-pulse 50-Hz bursts given every 200 ms (at 5 Hz), 2s on and 8s off) of intermittent theta burst (iTBS) excitatory TMS daily for 20 days (Monday–Friday for a 4-week period). The standard “Figure 8” Cool-B70 stimulation coil was centred over the scalp via the Beam F3 method (24). The sham group received the same intervention as the real group, except that the coil was flipped 180 degrees away from the skull. Resting motor threshold (MT) was defined as the stimulus strength over the thumb area of the motor cortex that produced five motor-evoked potentials responses of at least 50 microvolts (mV) in 10 trials. We measured MT before the first intervention and on every fifth intervention thereafter. Intensities of 100% MT were applied to all participants.
Visual analog scales (VAS) were administered to measure cue-induced craving. Cognitive function was assessed using a Chinese version of the CogState Battery, which includes the International shopping list task (ISL), Two back task (TWOB), Groton maze learning task (GML), Continuous paired association learning task (CPAL) and Social emotional cognition task (SEC). Five dimensions of cognitive function are measured by the CogState Battery: verbal learning and memory, working memory, problem solving/error monitoring, spatial working memory and social cognition. Ratings were performed at baseline and the day after completion of the 4-week course of intervention.
Outcomes were evaluated by experienced and trained psychiatrists, and neither psychiatrists nor participants knew whether the participants were receiving real or sham stimulation. Assessments of blinding and side effects were recorded at every session.
Image acquisition
All images were acquired on a Siemens Tim Trio 3T MRI scanner (Erlangen, Germany) equipped with a 12-channel head coil. High-resolution T1-weighted images were acquired (repetition time TR =2,300 ms, echo time TE =3 ms, flip angle FA=9°, number of slices=176, field of view FOV=256×256 mm2. MRS was acquired at baseline and the day after completion of the 4-week course of intervention.
GABA-edited 1H-MRS experiments were conducted using a MEGA-PRESS sequence (TE =68 ms, TR =1,800 ms, acquisition time 10 min 20 s, bandwidth 1200 Hz, 1024 datapoints, 160 water-suppressed, 8 water-unsuppressed averages). Voxels (20×20×20 mm3 =8 mL) were located in the left DLPFC (Figure 1). In addition to MEGA-PRESS spectra, regular point resolved spectroscopy (PRESS) spectra (TR =1,800 s, TE =35 ms, acquisition time =5 min 20 s) were also obtained from the same voxel and used for quantification of major metabolites including glutamate + glutamine (Glx), n-acetyl-aspartate (NAA), inositol (Ins), glycerophosphocholine + phosphocholine (GPC + PCh), phosphocreatine + creatine (PCr + Cr), etc. Spectra were analyzed using a linear combination model (LCModel version 6.3-0I) (LCMODEL Inc., CA). Shimming was performed to achieve a full-width at half maximum (FWHM) ≤12 Hz. Water unsuppressed spectra were also collected from the same voxel for absolute quantification. PRESS metabolite fits with a percentage standard deviation (%SD) value from LCModel over 20% were excluded from subsequent analysis. As the %SD of glutamine and glutamate were not stably reported by the LCModel, we used the combined signal of Glx, which is resolved at 2.1–2.4 and 3.65–3.8 ppm. GABA is resolved at 3.01 ppm. As there was no significant difference of NAA concentration between baseline and postintervention, the quantification of the brain metabolites was evaluated as the relative values to NAA resonance at 2.02 ppm.
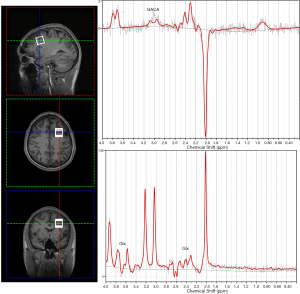
We used Gannet 3.0 (http://www.gabamrs.com), a MATLAB (The MathWorks Inc., Natick, MA) based software package, to create a mask of the voxel size and SPM12 to segment the T1-weighted image into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) based on the spatial coordinates in the scanner, to correct the neurometabolite levels for fraction of CSF in the DLPFC.
Statistical analysis
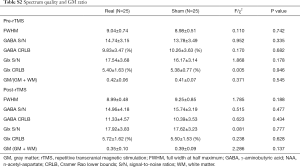
Analyses of variance (ANOVA) for continuous variables and chi-square tests for categorical variables were used to compare demographic and drug use characteristics and MRS spectrum quality indices: FWHM values, Cramer-Rao lower bounds (CRLB), signal-to-noise ratios (S/N) and GM ratios [GM/(GM+WM)] within DLPFC between real rTMS and sham rTMS groups at baseline.
Repeated measure ANOVA was used to assess the main effects of group (real rTMS vs. sham rTMS) and time (before and after stimulation). If there was a significant interaction between Group (active vs. sham) and Time (pre- vs. post-TMS), a simple effect test was used to confirm whether GABA/NAA and Glx/NAA are modulated by rTMS. In addition, Pearson or Spearman’s rho correlation coefficients between changes in neurometabolite levels and changes in craving and cognition scores were calculated. Significance level was thresholded at P<0.05. All statistical analyses were performed using SPSS Statistics version 16.
Results
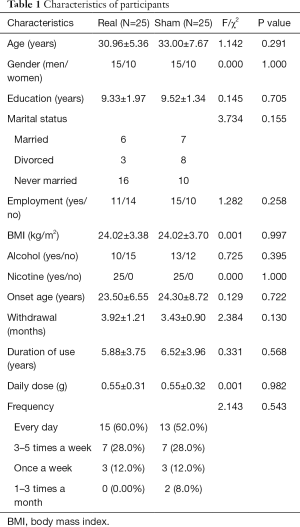
Table 1 shows the demographic and baseline clinical characteristics of the 50 patients (rTMS and sham group). No significant differences between the two groups were found.
Full table
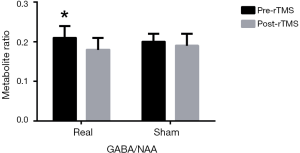
Before rTMS, the concentrations of all metabolites in real rTMS group were not statistically different from those in sham group (P>0.05). After four weeks intervention, for GABA/NAA, repeated ANOVA showed that there was no significant time × group interaction effect (F=0.158, P=0.695) but there was a significant difference for time effect (F=4.221, P=0.046). Simple effect tests showed that iTBS significantly reduced GABA/NAA (0.21±0.03 to 0.18±0.03, P=0.040), whereas sham did not (0.20±0.02 to 0.19±0.03, P=0.143) (Figure 2). The main effect showed there was no difference between two groups after intervention (F=0.270, P=0.608).
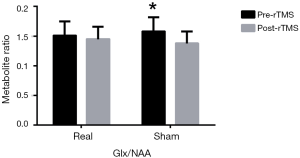
Glx/NAA showed that there was no significant time × group interaction effect (F=1.926, P=0.176) but there was a significant difference for time effect (F=8.878, P=0.005) and simple effect tests showed that iTBS did not reduce Glx/NAA significantly (1.51±0.24 to 1.45±0.21, P=0.458), whereas sham did (1.58±0.24 to 1.38±0.20, P=0.012) (Figure 3). There was no difference between the two groups after intervention (F=1.072, P=0.309).
Cue-induced craving ratings showed a significant time × group interaction effect (F=9.706, P=0.003) and a significant difference for time (F=14.282, P<0.001), and simple effect tests showed that iTBS significantly reduced craving (30.47±28.59 to 5.03±7.82, P<0.001), whereas sham did not (28.97±25.07 to 26.52±28.59, P=0.745). ISL scores showed that there was a significant time × group interaction effect (F=14.917, P<0.001) and simple effect tests showed that iTBS increased ISL correct scores (20.60±4.74 to 23.83±4.97, P=0.006), while sham stimulation did not (20.38±4.74 to 18.69±5.12, P=0.092). GML scores showed a significant difference for time (F=6.166, P=0.016) and simple effect tests showed that iTBS decreased GML error scores (71.93±27.14 to 59.80±23.35, P=0.003) but sham stimulation did not (79.97±32.10 to 73.86±29.48, P=0.205).
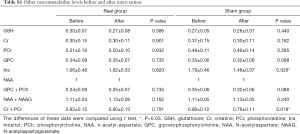
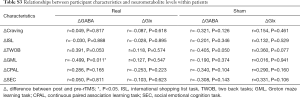
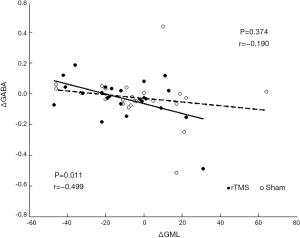
Other neurometabolite changes before and after intervention are shown in Supplementary Table S1. Spectrum quality and GM ratio are displayed in Supplementary Table S2. There were significant differences in Ins and Cr + PCr between two groups. Associations between changes in participants’ craving and cognition scores and neurometabolite levels are presented in Table S3. Change in GABA levels was correlated with the change in GML scores in the real stimulation group (Figure 4). GML scores increased as GABA levels decreased. No other correlations were found between clinical characteristics and neurometabolite levels.
Full table
Full table
Full table
Discussion
To our knowledge, this is the first study examining the effect of rTMS on brain GABA and Glx concentrations in methamphetamine dependent patients. Our results showed a declining trend for GABA/NAA levels in the DLPFC after intervention that was more apparent in the group who received real rTMS than in the sham stimulation group. Furthermore, cognitive function improvement was associated with the reduction of GABA levels in the DLPFC of patients who received real rTMS intervention. Previous studies have compared differences in brain metabolites between methamphetamine users and healthy subjects or other patients by using magnetic resonance spectroscopy (25-30). However, changes in GABA levels in target brain areas (e.g., DLPFC) after rTMS intervention for methamphetamine use disorder have not yet been studied.
Impairment in the prefrontal cortex is an important feature of addiction, which may result in executive dysfunction and uncontrolled drug use (31). As iTBS induces excitatory effects similar to those induced by high frequency rTMS, and given the close relationship between neurotransmitter flux and local brain activity, DLPFC activation is likely achieved by stimulating glutamatergic neurons or inhibiting GABAergic neurons. This is in agreement with our finding that GABA concentration of DLPFC was reduced in the patients who received active rTMS. In a recent meta-analysis, we found that low Glx levels in the DLPFC occur with substance use disorder (unpublished). Although we did not observe an increase of Glx in the real rTMS group, the decrease of Glx in sham group suggests that rTMS may induce a compensatory function.
As part of the executive control network, the prefrontal cortex is closely connected with many functional regions in the brain, particularly the anterior cingulate cortex (ACC) and insula (32,33). It has been hypothesized that rTMS action is propagated from the stimulation site to remote brain regions through excitatory and inhibitory interconnections. As a kind of interneuron, GABAergic neurons inhibit the over-activation of dopaminergic neurons and glutamatergic neurons. Methamphetamine is thought to aggravate these effects by interfering with the inhibitory function of GABAergic neurons that project from the prefrontal cortex to remote brain regions, leading to an excitatory-inhibitory imbalance (34). It has been shown that d-amphetamine administration increases the concentration of Glx in the dorsal ACC in volunteers (21,27). On the other hand, preclinical research has demonstrated that iTBS induces reductions in neuropeptide Y (NPY) and vesicular glutamate transporter type 1 (vGluT1) expression, which are involved in glutamatergic transmission and glutamateric presynaptic activity (35). TMS may exert similar metabolic effects in these regions.
The mechanism of the effect of rTMS on craving and cognitive function in methamphetamine dependent patients is still unclear. A possible explanation is discussed in our previous work (5). While we found no relationship between changes of craving and GABA or Glx, we revealed a significant correlation between changes in GABA and GML scores following active iTBS. The GML task taps into problem solving/error monitoring, with higher scores representing a higher number of errors. However, our results were somewhat confusing. The less GABA reduced, the more obvious cognitive function improved. Several mechanisms may underlie this phenomenon. Using resting-state functional MRI, functional connectivity analysis revealed that iTBS significantly reduced fronto-insular connectivity and GABA/Glx ratio in DLPFC, and GABA/Glx had a significant mediating effect on DLPFC-to-right anterior insula (rAI) connectivity changes (36). Although our results are not entirely consistent with this, it appears that iTBS induces a change in the inhibitiory/excitatory balance of the fronto-insular network based on circuits and neurochemistry. As a part of the salience network, the insula participates in executive control function together with the DLPFC. Another study reported that GABA in the striatum was associated with response inhibition, which is an important function of cognitive control (37). We assumed that GABA of prefrontal cortex was not directly involved in problem solving/error monitoring, but activated insula or striatum firstly, correlated negatively with metabolites or functional connections in these brain regions, producing inhibitory neural regulation, finally worked on executive function.
Some limitations of the present work should be noted. First, the small sample size may weaken the power to detect significant changes of the neurometabolites induced by rTMS. Second, only one brain region was measured, limiting our ability to explore the potential effect of rTMS on metabolite concentrations in other important regions [e.g., dACC, medial prefrontal cortex (mPFC), and insula]. Finally, it should be pointed out that not all patients respond to rTMS treatment. A recent study failed to find significant differences in GABA and Glx levels using iTBS targeting the inferior parietal lobe in healthy volunteers (Vidal-Piñeiro et al., 2015) (38), emphasizing the importance of individualized intervention. Evidence from neuroimaging studies for alterations in the brains of ATS users is still mixed (39,40). Therefore, conclusions should be drawn with caution. Future studies will require large sample sizes to clarify these issues.
In conclusion, we show prefrontal neurometabolites respond to rTMS in methamphetamine dependent patients. Our findings suggest that rTMS may reduce GABA/NAA levels in the left DLPFC of patients, and that decreases in GABA are related to the improvements in cognition. Larger studies are needed to further investigate the underlying modulation mechanisms of rTMS in patients with methamphetamine dependence, for example, the relationship between functional connectivity and metabolites changes in executive control network, and employing individualized plans when conducting rTMS intervention.
Acknowledgments
Thank all the participants for their contribution in this study.
Funding: The authors are supported by the National Key R&D Program of China (2018YFC0807400, 2017YFC1310400), National Nature Science Foundation (81771436, 81801319), Program of Shanghai Academic Research Leader (17XD1403300), Shanghai Municipal Health and Family Planning Commission (2017ZZ02021), Shanghai Key Laboratory of Psychotic Disorders (13DZ2260500), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), China Postdoctoral Science Foundation (2018M632137), Shanghai Jiao Tong University School of Medicine Innovation Funds for PhD Students (BXJ201844), Shanghai Clinical Research Center for Mental Health (19MC1911100), Clinical Research Center, Shanghai Jiao Tong University School of Medicine (DLY201818), Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ013), Program of Science and Technology Innovation Plan in Shanghai (18411961200), Shanghai Mental Health Center Flyer Plan (2016FX003). The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board and the ethics committee of Shanghai Mental Health Center (2016-11R). All participants participated voluntarily and provided written consent after understanding the whole procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernheim A, See R, Reichel C. Chronic methamphetamine self-administration disrupts cortical control of cognition. Neurosci Biobehav Rev 2016;69:36-48. [Crossref] [PubMed]
- Ashok AH, Mizuno Y, Volkow ND, et al. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA psychiatry 2017;74:511-9. [Crossref] [PubMed]
- Guo Q, Li C, Wang J. Updated Review on the Clinical Use of Repetitive Transcranial Magnetic Stimulation in Psychiatric Disorders. Neurosci Bull 2017;33:747-56. [Crossref] [PubMed]
- Grall-Bronnec M, Sauvaget A. The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: a critical literature review of efficacy, technical and methodological considerations. Neurosci Biobehav Rev 2014;47:592-613. [Crossref] [PubMed]
- Su H, Zhong N, Gan H, et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol Depend 2017;175:84-91. [Crossref] [PubMed]
- Mackey S, Allgaier N, Chaarani B, et al. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry 2019;176:119-28. [Crossref] [PubMed]
- Kearney-Ramos TE, Lench D, Hoffman M, et al. Gray and white matter integrity influence TMS signal propagation: a multimodal evaluation in cocaine-dependent individuals. Sci Rep 2018;8:3253. [Crossref] [PubMed]
- Li X, Du L, Sahlem G, et al. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend 2017;174:98-105. [Crossref] [PubMed]
- Hanlon CA, Dowdle LT, Moss H, et al. Mobilization of Medial and Lateral Frontal-Striatal Circuits in Cocaine Users and Controls: An Interleaved TMS/BOLD Functional Connectivity Study. Neuropsychopharmacology 2016;41:3032-41. [Crossref] [PubMed]
- Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci 2012;11:199-251. [Crossref] [PubMed]
- Lee MR, Denic A, Hinton DJ, et al. Preclinical (1)H-MRS neurochemical profiling in neurological and psychiatric disorders. Bioanalysis 2012;4:1787-804. [Crossref] [PubMed]
- Jiao D, Liu Y, Li X, et al. The role of the GABA system in amphetamine-type stimulant use disorders. Front Cell Neurosci 2015;9:162. [Crossref] [PubMed]
- Cadet JL, Jayanthi S. Epigenetics of methamphetamine-induced changes in glutamate function. Neuropsychopharmacology 2013;38:248-9. [Crossref] [PubMed]
- Caprioli D, Justinova Z, Venniro M, et al. Effect of Novel Allosteric Modulators of Metabotropic Glutamate Receptors on Drug Self-administration and Relapse: A Review of Preclinical Studies and Their Clinical Implications. Biol Psychiatry 2018;84:180-92. [Crossref] [PubMed]
- Phillips TJ, Reed C. Targeting GABAB receptors for anti-abuse drug discovery. Expert Opin Drug Discov 2014;9:1307-17. [Crossref] [PubMed]
- Sailasuta N, Abulseoud O, Hernandez M, et al. Metabolic Abnormalities in Abstinent Methamphetamine Dependent Subjects. Subst Abuse 2010;2010:9-20. [PubMed]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol 2008;3:165-72. [Crossref] [PubMed]
- Shanmugan S, Loughead J, Nanga R, et al. Lisdexamfetamine Effects on Executive Activation and Neurochemistry in Menopausal Women with Executive Function Difficulties. Neuropsychopharmacology 2017;42:437-45. [Crossref] [PubMed]
- Frye MA, Hinton DJ, Karpyak VM, et al. Elevated Glutamate Levels in the Left Dorsolateral Prefrontal Cortex Are Associated with Higher Cravings for Alcohol. Alcohol Clin Exp Res 2016;40:1609-16. [Crossref] [PubMed]
- Yoon JH, Grandelis A, Maddock RJ. Dorsolateral Prefrontal Cortex GABA Concentration in Humans Predicts Working Memory Load Processing Capacity. J Neurosci 2016;36:11788-94. [Crossref] [PubMed]
- Michael N, Gosling M, Reutemann M, et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci 2003;17:2462-8. [Crossref] [PubMed]
- Hone-Blanchet A, Mondino M, Fecteau S. Repetitive transcranial magnetic stimulation reduces anxiety symptoms, drug cravings, and elevates (1)H-MRS brain metabolites: A case report. Brain Stimul 2017;10:856-8. [Crossref] [PubMed]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci 2014;1327:79-93. [PubMed]
- Beam W, Borckardt J, Reeves S, et al. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul 2009;2:50-4. [Crossref] [PubMed]
- Howells FM, Uhlmann A, Temmingh H, et al. (1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res 2014;153:122-8. [Crossref] [PubMed]
- Burger A, Brooks S, Stein D, et al. The impact of acute and short-term methamphetamine abstinence on brain metabolites: A proton magnetic resonance spectroscopy chemical shift imaging study. Drug Alcohol Depend 2018;185:226-37. [Crossref] [PubMed]
- White TL, Monnig MA, Walsh EG, et al. Psychostimulant drug effects on glutamate, Glx, and creatine in the anterior cingulate cortex and subjective response in healthy humans. Neuropsychopharmacology 2018;43:1498-509. [Crossref] [PubMed]
- Crocker CE, Bernier DC, Hanstock CC, et al. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: a (1)H MRS study at 3Tesla. Schizophr Res 2014;157:231-7. [Crossref] [PubMed]
- Wu Q, Qi C, Long J, et al. Metabolites Alterations in the Medial Prefrontal Cortex of Methamphetamine Users in Abstinence: A (1)H MRS Study. Front Psychiatry 2018;9:478. [Crossref] [PubMed]
- Yang W, Yang R, Luo J, et al. Increased Absolute Glutamate Concentrations and Glutamate-to-Creatine Ratios in Patients With Methamphetamine Use Disorders. Front Psychiatry 2018;9:368. [Crossref] [PubMed]
- George O, Koob G. Control of craving by the prefrontal cortex. Proc Natl Acad Sci U S A 2013;110:4165-6. [Crossref] [PubMed]
- Sridharan D, Levitin D, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008;105:12569-74. [Crossref] [PubMed]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 2011;12:652-69. [Crossref] [PubMed]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex 1993;3:361-72. [Crossref] [PubMed]
- Jazmati D, Neubacher U, Funke K. Neuropeptide Y as a possible homeostatic element for changes in cortical excitability induced by repetitive transcranial magnetic stimulation. Brain Stimul 2018;11:797-805. [Crossref] [PubMed]
- Iwabuchi SJ, Raschke F, Auer DP, et al. Targeted transcranial theta-burst stimulation alters fronto-insular network and prefrontal GABA. Neuroimage 2017;146:395-403. [Crossref] [PubMed]
- Quetscher C, Yildiz A, Dharmadhikari S, et al. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct Funct 2015;220:3555-64. [Crossref] [PubMed]
- Vidal-Piñeiro D, Martín-Trias P, Falcón C, et al. Neurochemical Modulation in Posteromedial Default-mode Network Cortex Induced by Transcranial Magnetic Stimulation. Brain Stimul 2015;8:937-44. [Crossref] [PubMed]
- Müller F, Brandle R, Liechti ME, et al. Neuroimaging of chronic MDMA ("ecstasy") effects: A meta-analysis. Neurosci Biobehav Rev 2019;96:10-20. [Crossref] [PubMed]
- Bodea S. CNS metabolism in high-risk drug abuse: Insights gained from (1)H-, (31)P-MRS and PET. Radiologe 2018;58:34-9. [Crossref] [PubMed]


